��Ŀ����
��10�֣���ij��ѧ��ȤС�鰴���������̽��С���þ���Ͻ��Ʊ����������塱��ʵ�顣

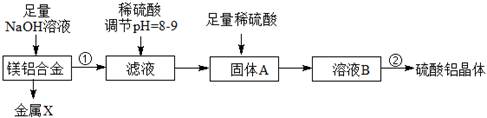
��þ���Ͻ��м�NaOH��Һ�Ļ�ѧ��Ӧ����ʽΪ ������X�� ��
����A�Ļ�ѧʽ �������ڰ����IJ���������Ũ���� �����ˡ����
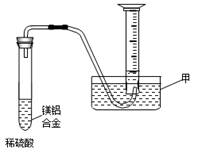
��ij��ȤС��Ϊ�ⶨþ���Ͻ��и���ɵ��������������ͼʾװ�á������������� ����Ҫ�ⶨ�������� ������֪��ʵ��ʱ���¶�ѹǿ��

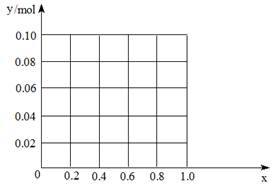
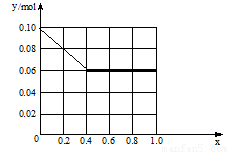
������һ������AlCl3��FeCl3�����Һ����֪����Al3+��Fe3+�����ʵ���֮��Ϊ0.10 mol���������Һ����170 mL 2mol/L NaOH��Һ����Al3+���ʵ����������ʵ����ı�ֵΪx����x=0.4ʱ�����������ʵ���Ϊ mol������ͼ�л�������������y mol����x��0��1���仯���ߡ�

���𰸡�
��10�֣���2Al+2NaOH+2H2O=2NaAlO2+3H2��, Mg, Al(OH)3 ,��ȴ�ᾧ�����½ᾧ��(4��)
��ˮ�� �������������þ���Ͻ���������¶ȡ�����ѹǿ���𣨵�ǰ2�����������֣���
��1+2�֣���3�֣�
��0.06mol ��ͼ��1+2�֣���3�֣�
��������

��ϰ��ϵ�д�
�����Ŀ