��Ŀ����
�������ƺ���������ƣ�Na2S2O3��������Ҫ�ĺ����ij��ѧ��ȤС����������й�ʵ�飬��д���пհף�
ʵ���֤��Na2SO3����Ԫ�صļ�̬�ܷ���
��
ת��
��1��ʵ���У�����Ҫ�õ����Լ���______�����ţ���
A��ϡ���� B��H2O2��Һ C��ϡ���� D�����۵⻯����Һ E��BaCl2��Һ
�۲쵽��������______��
ʵ�����ȡ��������ƾ���
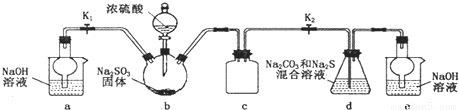
������ͼ��ʾװ�ý���ʵ�飮��֪������������������ᷢ����Ӧ
��װ��d�з�����Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2
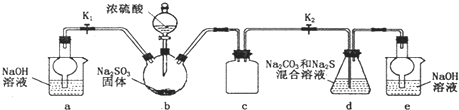
��2��װ��b�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��3��װ��c��������______��
��4��װ��d�еķ�Ӧ�������ȹرշ�Һ©���������������IJ�����______����d����Һ��ȴ��������������Ũ������ȴ���ᾧ�������������ƾ��壮
ʵ���ⶨij�����������Ʒ�Ĵ��ȣ��裺��Ʒ��ֻ��Na2SO3���ʣ�������·�����������ʵ�飺
��ȷ��ȡ�������������Ʒm g�����Ƴ�250mL������Һ��
����ȡ25.00mL������Һ����ƿ�У�����������ȩ����ֹNa2SO3��I2��Ӧ��������������Һ����n mol?L-1 I2����Һ�ζ���������Ӧ��2S2O32-+I2=S4O62-+2I-�������ζ��յ㣮
��5����������I2����ҺVmL����Ʒ��Na2S2O3�İٷֺ���Ϊ______���ô���ʽ��ʾ����
��6����ʵ�鷽�����ڵ�һ������ȱ����______��
ʵ���֤��Na2SO3����Ԫ�صļ�̬�ܷ���
| +4 |
| S |
| +6 |
| S |
��1��ʵ���У�����Ҫ�õ����Լ���______�����ţ���
A��ϡ���� B��H2O2��Һ C��ϡ���� D�����۵⻯����Һ E��BaCl2��Һ
�۲쵽��������______��
ʵ�����ȡ��������ƾ���
������ͼ��ʾװ�ý���ʵ�飮��֪������������������ᷢ����Ӧ
��װ��d�з�����Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2

��2��װ��b�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��3��װ��c��������______��
��4��װ��d�еķ�Ӧ�������ȹرշ�Һ©���������������IJ�����______����d����Һ��ȴ��������������Ũ������ȴ���ᾧ�������������ƾ��壮
ʵ���ⶨij�����������Ʒ�Ĵ��ȣ��裺��Ʒ��ֻ��Na2SO3���ʣ�������·�����������ʵ�飺
��ȷ��ȡ�������������Ʒm g�����Ƴ�250mL������Һ��
����ȡ25.00mL������Һ����ƿ�У�����������ȩ����ֹNa2SO3��I2��Ӧ��������������Һ����n mol?L-1 I2����Һ�ζ���������Ӧ��2S2O32-+I2=S4O62-+2I-�������ζ��յ㣮
��5����������I2����ҺVmL����Ʒ��Na2S2O3�İٷֺ���Ϊ______���ô���ʽ��ʾ����
��6����ʵ�鷽�����ڵ�һ������ȱ����______��
��1��֤��Na2SO3����Ԫ�صļ�̬�ܷ���+4�۱仯Ϊ+6�ۣ���Ҫ���������������������Ϊ��������ӣ�������������ӵĴ��ڣ�ѡ���������������Ĺ����������������������Ϊ�����ƣ�����ϡ�������������Ȼ������ɲ���������İ�ɫ������֤��������������ӣ���������������������Ӽ��飬���۵⻯������֤���������ʵ��Լ������ܼ�����������ӣ��ʴ�Ϊ��AD����Һ�г��ֲ���������İ�ɫ������
��2��װ��ͼ�з������ʵ��Ŀ�Ŀ�֪��װ��b��Ũ������������Ʒ�Ӧ���ɶ������������װ�ã���Ӧ�Ļ�ѧ����ʽΪ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
�ʴ�Ϊ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
��3��װ��c�ǰ�ȫƿ����������¶����������ֹ���������ã��ʴ�Ϊ����ֹ������
��4��������������Ⱦ������ʵ��������ŷŵ������У�װ��d�еķ�Ӧ�������ȹرշ�Һ©���������������IJ����ǹر�K2��K1���ʴ�Ϊ���ر�K2��K1��
��5����ȡ25.00mL������Һ����ƿ�У�����������ȩ����ֹNa2SO3��I2��Ӧ��������������Һ����n mol?L-1 I2����Һ�ζ���������Ӧ��2S2O32-+I2=S4O62-+2I-�������ζ��յ㣬�������ӷ���ʽ���м���õ�25ml��������������ʵ�����
2S2O32-+I2=S4O62-+2I-��
2 1
n��Na2S2O3�� nmol/L��V��10-3L
n��Na2S2O3��=2nV��10-3mol��
���Ƶ�250ml��Һ�к���n��Na2S2O3��=0.02nVmol��
��Ʒ��Na2S2O3�İٷֺ���=
��100%=
��100%���ʴ�Ϊ��
��
��6����ʵ��������֪�����µļ��飬ȱ�ٶ���ʵ�飬�ʴ�Ϊ��ȱ��ƽ��ʵ�飮
��2��װ��ͼ�з������ʵ��Ŀ�Ŀ�֪��װ��b��Ũ������������Ʒ�Ӧ���ɶ������������װ�ã���Ӧ�Ļ�ѧ����ʽΪ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
�ʴ�Ϊ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
��3��װ��c�ǰ�ȫƿ����������¶����������ֹ���������ã��ʴ�Ϊ����ֹ������
��4��������������Ⱦ������ʵ��������ŷŵ������У�װ��d�еķ�Ӧ�������ȹرշ�Һ©���������������IJ����ǹر�K2��K1���ʴ�Ϊ���ر�K2��K1��
��5����ȡ25.00mL������Һ����ƿ�У�����������ȩ����ֹNa2SO3��I2��Ӧ��������������Һ����n mol?L-1 I2����Һ�ζ���������Ӧ��2S2O32-+I2=S4O62-+2I-�������ζ��յ㣬�������ӷ���ʽ���м���õ�25ml��������������ʵ�����
2S2O32-+I2=S4O62-+2I-��
2 1
n��Na2S2O3�� nmol/L��V��10-3L
n��Na2S2O3��=2nV��10-3mol��
���Ƶ�250ml��Һ�к���n��Na2S2O3��=0.02nVmol��
��Ʒ��Na2S2O3�İٷֺ���=
| 0.02nV��158g/mol |
| m |
| 3.16nv |
| m |
| 3.16nv |
| m |
��6����ʵ��������֪�����µļ��飬ȱ�ٶ���ʵ�飬�ʴ�Ϊ��ȱ��ƽ��ʵ�飮

��ϰ��ϵ�д�
�����Ŀ


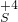 ��
��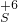 ת��
ת��
 ��
��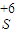 ת��
ת��