��Ŀ����
��2L�ܱ�������ͨ��amol ����A��bmol����B����һ�������·�����Ӧ��
xA(g)+yB(g) pC(g)+qD(g)
��֪��ƽ����Ӧ����vC��vA/2����Ӧ2min ʱ��A�����ʵ���������1/3��B�����ʵ���������a/2 mol����a molD���ɡ������ش�
��1����Ӧ2min�ڣ�v��A�� =��
��2����ѧ����ʽ�У�x��������������y����������������������p����������������������q������������������
��3��ƽ��ʱ��DΪ 2a mol����ʱB�����ʵ���Ũ��Ϊ��
����5�֣�
��1��a/12mol��L-1��min��1 ��2��2 3 1 6��3����b-a��/2mol��L-1
����:

��2L�ܱ�������ͨ��amol ����A��bmol����B����һ�������·�����Ӧ��xA(g)+yB(g) ![]() pC(g)+qD(g)��
pC(g)+qD(g)��
��֪��ƽ����Ӧ����vC��1/2vA����Ӧ2min ʱ��A��Ũ�ȼ�����1/3��B�����ʵ���������a/2mol����a mol D���ɡ�
�ش��������⣺
��1����ѧ����ʽ�У�x�� ��y�� ��p�� ��q�� ��
��2����Ӧ2min�ڣ�vA = ��
��3����Ӧƽ��ʱ��DΪ 2amol����B��ת����Ϊ ��
��4����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
| �¶ȡ� | 1000 | 1150 | 1300 |
| ƽ�ⳣ�� | 4.0 | 3.7 | 3.5 |


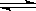 2NH3(g)����֪����Ӧ2min�ﵽƽ�� ����ʱN2��Ũ�ȼ�����2/3��
2NH3(g)����֪����Ӧ2min�ﵽƽ�� ����ʱN2��Ũ�ȼ�����2/3��