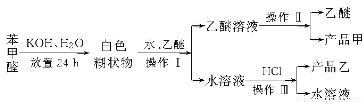
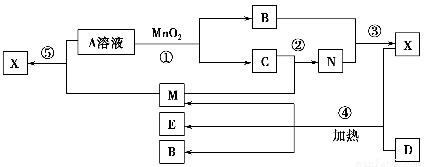
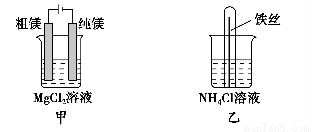
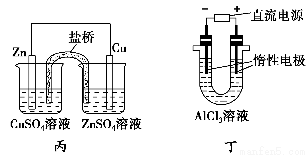
��Ŀ����
����ת����ϵ����X��Y����������;�㷺�����ֽ������ʣ�A��B����������A�ʺ���ɫ��C��D��E����ѧ���������ֻ��������ת����ϵ�ش����⣺
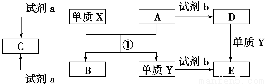
��1����д����Ӧ���Ļ�ѧ����ʽ___________________________________________��
��2������D��Һ��Y���ӵķ�����_____________________________________��
��3�����Լ�a��NaOH��Һ��д������X��NaOH��Һ��Ӧ�����ӷ���ʽ______________________________��
��4�����Լ�b��H2SO4����ҵ����E��H2SO4��NaNO2Ϊԭ����ȡ��Ч��ˮ��Y��OH��SO4����֪��ԭ����ΪNO����÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________________________��
��5����ҵ�ϵ�����ڵ�B��ȡXʱ�������������������ڱ�״���µ����Ϊ33.6 m3�����������������Ϊ________kg��
��1��Fe2O3��2Al 2Fe��Al2O3
2Fe��Al2O3
��2��ȡ����D��Һ���Թ������μӼ���KSCN��Һ������Һ���ɫ����֤����Fe3��
��3��2Al��2OH����2H2O=2AlO2-��3H2��
��4��2FeSO4��H2SO4��2NaNO2=2Fe��OH��SO4��Na2SO4��2NO��
��5��54
����������������AΪFe2O3��XΪ����ͨ�����ȷ�Ӧ������������B������������Y����D��Fe��Ӧ����E�����Լ�bΪ������D��E�ֱ������κ������Ρ���4��EΪFeSO4�����Ļ��ϼ�Ϊ��2�ۣ���Fe��OH��SO4�����Ļ��ϼ�Ϊ��3�ۣ�����NaNO2�����Ļ��ϼ�Ϊ��3�ۣ���NO�����Ļ��ϼ�Ϊ��2�ۣ������ݵ��ӵ�ʧ�غ㡢�����غ㶨�ɼ�����ƽ��Ӧ��ѧ����ʽ����5��������Ӧ��2O2����4e��=O2����33.6 m3���������ʵ���Ϊ1 500 mol��ת�Ƶ�����Ϊ6 000 mol��������Ӧ��Al3����3e��=Al���ʿ��Եõ�2 000 mol��Al������Ϊ��2 000 mol��27 g��mol��1��54 000 g��54 kg��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�