��Ŀ����
ij����С����е�ⱥ��ʳ��ˮ��ʵ��(��ͼ)����ش��������⡣

(1)ͨ��һ��ʱ��ɹ۲쵽_________�缫(���������������)������Һ��ɫ�ȱ�죬�õ缫�ϵĵ缫��ӦʽΪ____________________________________��
(2)����Һ��ⷴӦ�Ļ�ѧ����ʽΪ______________________________________________��
(3)����ʼʱ���������������������ĵ缫��ӦʽΪ________________________________��
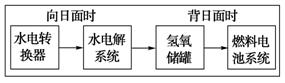
(4)����H2��O2Ϊ��Ӧ���KOHΪ�������Һ���ɹ�������ȼ�ϵ��(��ͼ)�������缫���ɶ����̿�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ硣����a��__________��(���������)��b���ĵ缫��ӦʽΪ______________________________��

(1)���� 2H++2e-![]() H2��
H2��
(2)2NaCl+2H2O Cl2��+H2��+2NaOH
Cl2��+H2��+2NaOH
(3)Fe-2e-![]() Fe2+
Fe2+
(4)�� O2+2H2O+4e-![]() 4OH-
4OH-
�����������ڵ�ⱥ��ʳ��ˮ������H+�ŵ磬ʹH2O![]() H++OH-��ƽ�����������ƶ�������������OH-�������������ȱ�졣�����������������Ϊ���ý������缫�����ȷŵ硣(4)����ԭ���ԭ�������ϼ۽��͵�H2���ڵļ�ӦΪ������O2�����������õ��ӵĻ�ԭ��Ӧ��
H++OH-��ƽ�����������ƶ�������������OH-�������������ȱ�졣�����������������Ϊ���ý������缫�����ȷŵ硣(4)����ԭ���ԭ�������ϼ۽��͵�H2���ڵļ�ӦΪ������O2�����������õ��ӵĻ�ԭ��Ӧ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ