��Ŀ����
���ᣨHCOOH����һ���д̼���ζ����ɫҺ�壬�к�ǿ�ĸ�ʴ�ԡ��۵�8.4�棬�е�100.7�棬����ˮ���Ҵ����ܣ�������160�漴�ֽ�ɶ�����̼��������
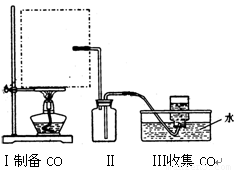
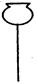
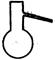
��1��ʵ���ҿ��ü�����Ũ���Ṳ���Ʊ�һ����̼��HCOOHŨ����========H2O+CO����ʵ��IJ���װ������ͼ��ʾ���Ʊ�ʱ�ȼ���Ũ������80�桪90�棬����ε�����ᡣ

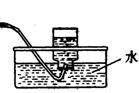
���Ʊ�CO �� ���ռ�CO
�ٴ���ͼ��ѡ���������������������ȱ�����巢��װ�ã����ӱ�Ҫ�����ӡ������ܡ���Ƥ�ܣ��̶�װ�ò��û����������������е��Լ���
![]()
![]()
![]()
![]()
��Һ©�� ����©�� ������ƿ ����ƿ �¶ȼ�
��װ�â�������� ��
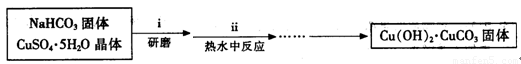
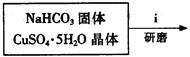
��2��ʵ���ҿ��ü����Ʊ�����ͭ���䷽������������ͭ��̼�����������Ƶü�ʽ̼��ͭ��Ȼ�����������Ƶ���ˮ����ͭ[Cu��HCOO��2��4H2O]���塣��صĻ�ѧ����ʽ�ǣ�
2CuSO4+4 NaHCO3== Cu��OH��2��CuCO3��+3CO2��+2Na2SO4+H2O
Cu��OH��2��CuCO3+4HCOOH+ 5H2O==2 Cu��HCOO��2��4H2O+ CO2��
ʵ�鲽�����£�
��ʽ̼��ͭ���Ʊ���
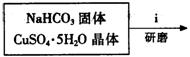
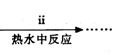
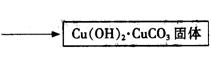
�۲��袡�ǽ�һ����CuSO4��5H2O�����NaHCO3����һ��ŵ��в�����ĥ����Ŀ���� ��
�ܲ��袢���ڽ����½���������ֶ�λ���������ˮ�У���Ӧ�¶ȿ�����70�桪80�棬������� ����дʵ������˵���¶ȹ��ߡ�
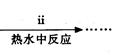
����ͭ���Ʊ���
��Cu��OH��2��CuCO3��������ձ��У�����һ�����ȵ�����ˮ������μ����������ʽ̼��ͭǡ��ȫ���ܽ⣬���ȹ��˳�ȥ�������������ʡ���ͨ�����������Һ��ԭ�����1/3ʱ����ȴ�������壬���ˣ�����������ˮ�Ҵ�ϴ�Ӿ���2��3�Σ����ɣ��õ���Ʒ��
�ݡ����ȹ��ˡ��У����롰���ȡ���ԭ���� ��
�����Ҵ�ϴ�Ӿ����Ŀ���� ��
�٣�2�֣�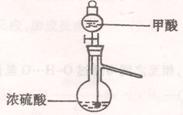 ����ѡ����ȷ����ʱҺ��1�֣��¶ȼ�ˮ�����λ��1��
����ѡ����ȷ����ʱҺ��1�֣��¶ȼ�ˮ�����λ��1��
�ڷ�ֹˮ���е�ˮ��������������ƿ�У�2�֣�
����ϸ����Ͼ��ȣ���1�֣���2�֣�
�ܳ��ֺ�ɫ���壨2�֣�
�ݷ�ֹ����ͭ����������2�֣�
��ϴȥ��������ˮ���������ʣ�2�֣�
����:
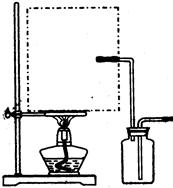
����Ϊ�Ǹ�Һ������Ʊ����壬��Ҫ�����¶ȣ������巢��װ����ʵ��������ϩ�ķ���װ��һ����������ͼ��ʾ������Ϊ������ˮ���ռ����壬��������Ȳ����Ⱦ��п��ܷ����������ʢ�Ϊ������װ�ã�����Ϊ��ȡ������μ�����ˮ�У������ֹ���Ҫ��Ͼ��ȣ���NaHCO3�����CuSO4��H2O�������ɿ飬��Ҫ��ϸ�ſɻ�;��ȣ���������ͭ��̼��ͭ���Ⱦ����ȶ��ֽ⣬ǰ�����ɺ�ɫ������ͭ��ˮ�������ߺ�ɫ������ͭ�Ͷ�����̼�������¶ȹ���ʹ���Ƿֽ⣬���ֺ�ɫ���壻������Ũ�������ͭ��Ũ�ȱȽϸߣ������ȴ�����»��Ծ������ʽ��������Ҫ�����ȡ����ˣ�����Һ��ȴ�ᾧ�������ľ������һ�����������Һ�е��������ʣ����������ˮ������һЩ���������ʶ��п��ܲ��������棬�����Ҵ�ϴ�Ӿ����Ŀ����ϴȥ��������ˮ���������ʡ�

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д�





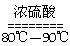 H2O+CO����
H2O+CO����