��Ŀ����
�л���A����������ˮ�������㾫��A�DF�ɷ�������ת����B��C����Է���������ȣ�B�����к���2������D����������ϵ�һ��ȡ������3�֣�F��ʹBr2�����Ȼ�̼��Һ��ɫ��

�Իش�
��1��E�еĹ����������� ��
|
a��������Ӧ b���ӳɷ�Ӧ c����ȥ��Ӧ d��������Ӧ
��3��A�Ľṹ��ʽΪ ��C��F�Ļ�ѧ����ʽΪ ��
��4��д��ˮ��ʱ�������������ҷ����к���3������A��ͬ���칹��ṹ��ʽ
��
��5����B��C�Ļ����5mol��������O2����ȫȼ�գ�����CO2492.8L����״��������������B�����ʵ���Ϊ mol��
��1���ǻ�
��2��a b
![]()
![]() ��3��CH3CHCOOCH2CH2CHCH3
��3��CH3CHCOOCH2CH2CHCH3
![]() CH3 CH3
CH3 CH3
![]()
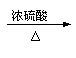 CH3CHCH2CH2OH CH3�DCH�DCH=CH2+H2O
CH3CHCH2CH2OH CH3�DCH�DCH=CH2+H2O
CH3 CH3
![]() ��4��CH3CH2CH2CH2COOCH2CHCH3
��4��CH3CH2CH2CH2COOCH2CHCH3
CH3
![]() CH3CH2CH2CH2COOCHCH2CH3
CH3CH2CH2CH2COOCHCH2CH3
CH3
��5��3

��1������ʵ��������ʵ����ʵ����������ȷ���� ������ţ���
A����������ƽ����17.55g�Ȼ��ƾ���
B��̼������Һ�����ڴ����������Լ�ƿ
C���ø����pH��ֽ�ⶨ������ˮ��pH
D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ��
E����FeCl3��Һ�еμ�����NaOH��Һ������ȡFe(OH)3����
F����ȥCO2�����л��е�����HCl�����Խ�����ͨ�뱥��̼��������Һ
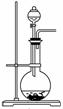
��2����ͼΪ��ѧ��ѧʵ���г�����ʵ��װ��



A B C
ʵ���ҳ���װ��A�Ʊ��±������壬�뽫��Һ©����Բ����ƿ��Ӧװ�Ļ�ѧ�Լ���д������
|
���� |
O2 |
Cl2 |
NH3 |
|
��Һ©�����Լ� |
|
|
Ũ��ˮ |
|
Բ����ƿ���Լ� |
|
KMnO4 |
|
����Bװ����Һ�ռ����壬����Ӧ�Ӹ�װ��________(����ҡ�)�ܿڵ������������ø�װ���ռ�Cl2���Լ�ƿ��ʢ�ŵ��Լ�Ϊ ��
Cװ�����ڴ�����������Ի�������Ⱦ�������ø�װ������Cl2����ʱ�ձ��з�����Ӧ�����ӷ���ʽΪ ��������װ���н���ʢ��ϡ���ᣬͨ�����ʺ����հ�����ԭ���� �������ձ��ж����ټ���һ��Һ̬�л�����ɰ�ȫ���հ����������л���Ϊ ��
��1������ʵ��������ʵ����ʵ��������ȷ���� ��
A����������ƽ����17.55g�Ȼ��ƾ���
B��̼������Һ�����ڴ��������Լ�ƿ
C���ø����pH��ֽ�ⶨ������ˮ��pH
D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ��
E����FeCl3��Һ�еμ�����NaOH��Һ������ȡFe(OH)3����
F���ñ���ȡ��ˮ�е���ʱ���ӷ�Һ©���¿ڷų�����ı���Һ
��2����ͼΪ��ѧ��ѧʵ���г�����ʵ��װ��
![]()
![]()
A B C
�� ʵ���ҳ���װ��A�Ʊ����壬�뽫��Һ©����Բ����ƿ��Ӧװ�Ļ�ѧ�Լ���������д���±��С�
| ���� | O2 | Cl2 | NH3 |
| ��Һ©�����Լ� | H2O | Ũ��ˮ | |
| Բ����ƿ���Լ� | KMnO4 |
�� ����Bװ����Һ�ռ����壬����Ӧ�Ӹ�װ��________������ҡ����ܿڵ������������ø�װ���ռ�Cl2���Լ�ƿ��ʢ�ŵ��Լ�Ϊ ��
�� Cװ�����ڴ�����������Ի�������Ⱦ�������ø�װ������Cl2����ʱ�ձ��з�����Ӧ�����ӷ���ʽΪ ��������װ���н���ʢ��ϡ���ᣬͨ�����ʺ����հ�����ԭ���� �������ձ��ж����ټ���һ��Һ̬�л�����ɰ�ȫ���հ����������л���Ϊ ��