��Ŀ����
�������ƾ��壨CaO2��8H2O���ʰ�ɫ������ˮ��������350�����ҿ�
ʼ�ֽ�ų��������������ƿ����ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ��Ӧ����
���ȡ�ʵ���ҿ��ù�ҵ̼��ƣ���MgCO3��FeCO3�����ʣ���ȡ������̼��ƣ�Ȼ����
�ô���̼�����ȡ�������ƣ�����Ҫ�������£�
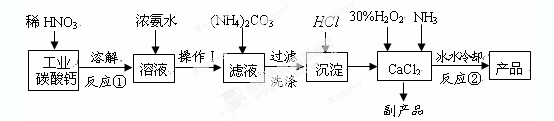
�ش��������⣺
�����������漰�Ļ�ѧ��Ӧ����������ԭ��Ӧ���� ������д����������һ�������ӷ���ʽ�� ��
��2����Ӧ������CaO2��8H2O�Ļ�ѧ��Ӧ����ʽΪ ��
��Ӧʱ�ñ�ˮ��ȴ����Ҫԭ���� ��
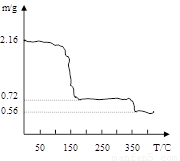
��3�����������ƾ����������м��Ȳ��������¶ȡ������Ʒ�������¶ȵı仯��ͼ������ʾ����350���Ժ����ù������ʵĻ�ѧʽΪ ��
��1��3FeCO3+10H++NO3-=3Fe3++NO��+3CO2��+5H2O
��2��CaCl2+H2O2+2NH3+8H2O=CaO2��8H2O+2NH4Cl
��ֹH2O2�ֽ⣬����������ʻ�CaO2��8H2O�ܽ�ȣ���߲���
��3��CaO
��������
�����������1��ֻ�з�Ӧ���е�FeCO3�����ᷴӦ��������ԭ��Ӧ����ӦΪ
3FeCO3+10H++NO3-=3Fe3++NO��+3CO2��+5H2O
��2���÷�Ӧ�ķ�Ӧ��ΪCaCl2��H2O2��NH3���ʷ���ʽΪCaCl2+H2O2+2NH3+8H2O=CaO2��8H2O+2NH4Cl
��ΪH2O2�����ֽ⣬�ʲ��ñ�ˮ��ȴ�ķ�ʽ��ֹ��ֽ⣬��������ʣ�ͬʱ�¶ȵ��ܽϵ��ܽ�ȣ���߲��ʡ�
��3���ڼ��ȹ����й����и�Ԫ�ص������䣬������2.16g���ȵ�350���Ϊ0.56g��
n(Ca)=2.16��216=0.01mol m(CaO) =0.01��56=0.56g �ʸù���ΪCaO
�������Ľⷨ��һ�ֲ²⣬��������ȼ���ʧȥȫ���ᾧˮʱ���������ɴ˼���������ó���ѧʽ��
���㣺���黯�������з�Ӧ��ע�������Ӧԭ�����й�ͼ��ļ�����й����⡣

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�