��Ŀ����
��ͼ�ٱ�ʾ��MgCl2��AlCl3��NH4Cl�������ʵĻ����Һ����μ���NaOH��Һʱ�����������ʵ�����NaOH��Һ�������ϵ��ͼ�ڱ�ʾij��Ӧ�����е�������ϵ�����и�������ʾ��ͼ��һ�µ���
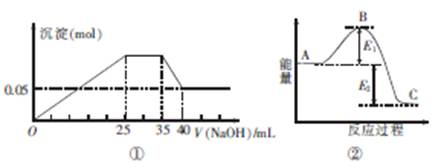
- A.ͼ�����������ӵ����ʵ���֮��n��Mg2+����n��Al3+����n��NH+4��=1��1��2
- B.ͼ����ʹ�õ�NaOH��Ũ��Ϊ2 mol��L-1
- C.ͼ������ʹ�ô�����B��ή��
- D.ͼ������������C��Ӧ��������A����Ӧ�Ļ��ΪE1+E2
B
����ͼ��0��25mL��AL3+��Mg2+�����ĵ�NaOH;25��35mL��NH4+�����ĵ�NaOH��35��40mL��AL(OH)3�����ĵ�NaOH��Mg(OH)2���������ʵ���Ϊ0.05mol��
�����ӷ���ʽ��Mg2++2OH-=Mg(OH)2����AL3++3OH-=AL(OH)3����NH4++OH-=NH3��H2O��
AL(OH)3+OH-=ALO2-+2H2O����֪��AL(OH)3����NaOH��Һ5mL����AL3+��������NaOH��Һ15 mL��Mg2+����NaOH��Һ10mL�����Կ����n��Mg2+����n��Al3+����n��NH+4��=1��1��2A�ԣ�c��NaOH��="10" mol��L-1��B����ʹ�ô����ή�ͷ�Ӧ����Ļ�ܣ�B�㽵�ͣ�C�ԣ�D�ԡ�
����ͼ��0��25mL��AL3+��Mg2+�����ĵ�NaOH;25��35mL��NH4+�����ĵ�NaOH��35��40mL��AL(OH)3�����ĵ�NaOH��Mg(OH)2���������ʵ���Ϊ0.05mol��
�����ӷ���ʽ��Mg2++2OH-=Mg(OH)2����AL3++3OH-=AL(OH)3����NH4++OH-=NH3��H2O��
AL(OH)3+OH-=ALO2-+2H2O����֪��AL(OH)3����NaOH��Һ5mL����AL3+��������NaOH��Һ15 mL��Mg2+����NaOH��Һ10mL�����Կ����n��Mg2+����n��Al3+����n��NH+4��=1��1��2A�ԣ�c��NaOH��="10" mol��L-1��B����ʹ�ô����ή�ͷ�Ӧ����Ļ�ܣ�B�㽵�ͣ�C�ԣ�D�ԡ�

��ϰ��ϵ�д�
�����Ŀ
��ͼ�ٱ�ʾ��MgCl2��AlCl3��NH4Cl�������ʵĻ����Һ����μ���NaOH��Һʱ�����������ʵ�����NaOH��Һ�������ϵ��ͼ�ڱ�ʾij��Ӧ�����е�������ϵ�����и�������ʾ��ͼ��һ�µ���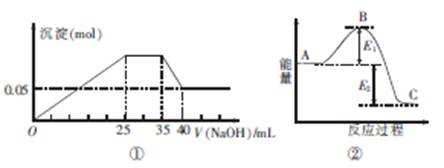
| A��ͼ�����������ӵ����ʵ���֮��n��Mg2+����n��Al3+����n��NH+4��=1��1��2 |
| B��ͼ����ʹ�õ�NaOH��Ũ��Ϊ2 mol��L-1 |
| C��ͼ������ʹ�ô�����B��ή�� |
| D��ͼ������������C��Ӧ��������A����Ӧ�Ļ��ΪE1+E2 |

