��Ŀ����
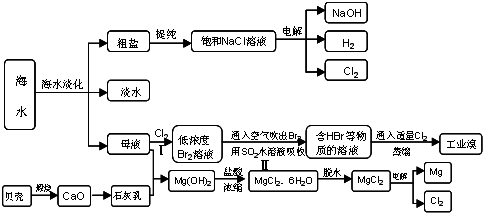
��ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��������������⣺
(1)��ˮ�к��д������Ȼ��ơ��Ȼ����еĽ���Ԫ�ػ��ϼ�Ϊ___________�ۡ�
(2)Ŀǰ������ʵ�õġ���ˮ��������Ҫ����֮һ�����������ǽ���ˮ�������������������ȴ���øߴ��ȵ�ˮ���ɴ˿��ж�������___________��������仯����ѧ�仯������
��3����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ����ӦʽΪ��
ʳ��+H2O![]() NaOH+H2+Cl2��δ��ƽ�����÷�Ӧ��ʳ�εĻ�ѧʽ��_____________________�����õ������������36.5%��Ũ����100 t��������Ҫ����ʳ��___________t��
NaOH+H2+Cl2��δ��ƽ�����÷�Ӧ��ʳ�εĻ�ѧʽ��_____________________�����õ������������36.5%��Ũ����100 t��������Ҫ����ʳ��___________t��
(4)���������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷����������£�
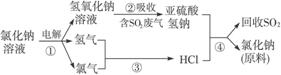
д���٢۵Ļ�ѧ��Ӧ����ʽ��__________________________��__________________________��
(1)+1
(2)�����仯
��3��NaCl 58.5
(4)2NaCl+2H2O![]() H2��+Cl2��+2NaOH H2+Cl2
H2��+Cl2��+2NaOH H2+Cl2![]() 2HCl
2HCl
����:
��1�����ڻ���������+1�ۡ���2����������ֻ��ˮ��״̬��Һ̬����̬�ٵ�Һ̬�Ĺ��̣��������������仯����3���������غ㶨�ɿ�֪��ʳ�εĻ�ѧʽΪNaCl������ԭ���غ��֪��������Ҫ����ʳ�Σ�36.5%��100 t��x=36.5��58.5��x=58.5 t��

 ��У����ϵ�д�
��У����ϵ�д�