��Ŀ����
���Ṥҵ�����е�β�����ô�����Һ���գ��йصĻ�ѧ��ӦΪ��2NO2+Na2CO3��NaNO2+NaNO3+CO2�� ��
NO+NO2+Na2CO3��2NaNO2+CO2�� ��
(1)���ݷ�Ӧ�٣�ÿ����22.4 L(��״��)CO2������Һ����������__________g��
(2)����1 000 g��������Ϊ21.2%�Ĵ�������Һ����Na2CO3��10H2O���ٿˣ�
(3)����1 000 g��������Ϊ21.2%�Ĵ�������Һ���������Ṥҵβ����ÿ����22.4 L(��״��)CO2ʱ������Һ����������44 g��
�ټ�������Һ�е�NaNO2��NaNO3���ʵ���֮�ȡ�
��1 000 g��������Ϊ21.2%�Ĵ�����20 �澭����������Ṥҵβ����������688 g ˮ����ȴ��0 �棬��������NaNO2_________________�ˣ�(0 ��ʱ��NaNO2���ܽ��Ϊ71.2 g)
(1)48 g (2)572 g
(3)��n(NaNO2)��n(NaNO3)=5��3
��101.3 g
������(1)���ݲ���������
2NO2+Na2CO3====NaNO2+NaNO3+CO2�� ��m
1 mol 48 g
![]() x g
x g
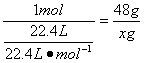 x=48
x=48
(2)�������⣺��m(Na2CO3��10H2O)=![]() ��286 g��mol-1=572 g
��286 g��mol-1=572 g
(3)��2NO2+Na2CO3��NaNO2+NaNO3+CO2�� ��m=48 g
NO+NO2+Na2CO3��2NaNO2+CO2�� ��m=32 g
����NO2�봿�Ӧ������CO2���ʵ���Ϊa����NO��NO2�봿�Ӧ������CO2���ʵ���Ϊb��
![]()
���![]()
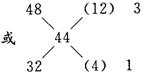
��n(NaNO2)��n(NaNO3)=5��3
�������ɵ�n(NaNO2)Ϊ5x��n(NaNO3)Ϊ3x��
��Na+�غ㣺5x+3x=4 mol��x=0.5 mol
m(NaNO2)=5��0.5 mol��69 g��mol-1=172.5 g
m(H2O)��=1 000 g��78.8%-688 g=100 g
����m(NaNO2)(���)=172.5 g-71.2 g=101.3 g

 ��������ϵ�д�
��������ϵ�д�