��Ŀ����
������A��Է�������Ϊ86,̼����������Ϊ55.8%,��Ϊ7.0%,����Ϊ����A����ط�Ӧ����ͼ��ʾ: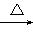
��֪R��CH==CHOH(ϩ��)���ȶ�,�ܿ�ת��ΪR��CH2CHO��
����������Ϣ�ش���������:
(1)A�ķ���ʽΪ_______________________;
(2)��Ӧ�ڵĻ�ѧ����ʽ��_______________________;
(3)A�Ľṹ��ʽ��_______________________;
(4)��Ӧ�ٵĻ�ѧ����ʽ��_______________________;
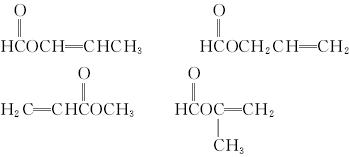
(5)A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ��(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ��___________��____________��____________��____________��
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ_________��
��1��C4H6O2
(2)CH3CHO+2Cu(OH)2![]() CH3COOH+Cu2O+2H2O
CH3COOH+Cu2O+2H2O
(3)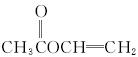
(4)
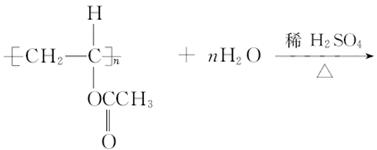
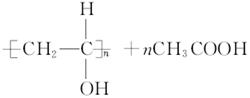
(5)
(������ȷ��Ҳ�ɣ�
��6��HOCH2��C�ԡ�C��CH2OH
����:�ɻ�����A����Է�������Ϊ86,̼����������Ϊ55.8%,��Ϊ7.0%,����Ϊ���ɼ��������ʽΪC4H6O2��A����ˮ��˵�����������������ֿ��Ծۺ�˵�����в�����̼̼˫��������ͼ���Կ���ˮ����������ת��������֪CDE��Ϊ2��̼ԭ�ӣ���DΪCH3COOH��AΪCH3COOCH====CH2��

 ������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ��
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ��








