��Ŀ����
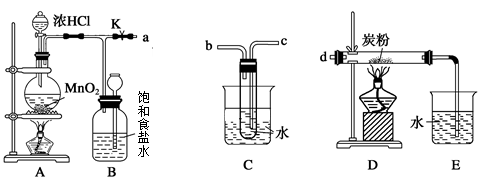
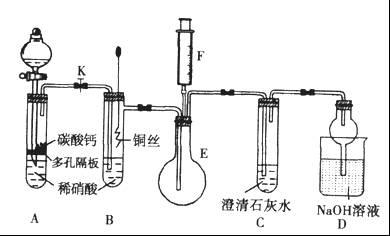
ij��ѧѧϰС��ͬѧ����ʵ�������е���ȡ������ҩƷ���������ͼ��ʾ��ʵ��װ�ã����ּг�����δ����������ȡ��̽�������Ļ�ԭ�ԡ����鷴Ӧ�����ش��������⣺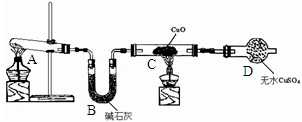
��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2��B�м�ʯ�ҵ������� ��
��3��C�к�ɫ�����죬�Ҳ���������Կ�������Ⱦ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
D�����������________________________________________________________________________��
��4����װ�ô�������ȱ�ݣ���ȱ���� ��
��5����ҵ�г��õ����������ڸ��¡���ѹ������ý�������������ºϳɰ�������С��ͬѧģ�������Ҳ�ϳɳ��˰�������֪��ʼʱ����2 mol N2��6 mol H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ������5 min�������������ʵ���������1 mol���������ʱ������H2��ʾ�Ļ�ѧ��Ӧ����
Ϊ ��
��1��Ca(OH)2��2NH4Cl CaCl2��2NH3����2H2O��2�֣���
CaCl2��2NH3����2H2O��2�֣���
��2�����ﰱ����1�֣���
��3��3CuO+2NH3  3Cu+N2+3H2O��2�֣�����ˮ����ͭ������1�֣���
3Cu+N2+3H2O��2�֣�����ˮ����ͭ������1�֣���
��4��ȱ��β������װ�ã�1�֣���
��5��0.15 mol/(L��min)��2�֣�
���������������1��װ��A���Ʊ������ģ�������з�����Ӧ�Ļ�ѧ����ʽ��Ca(OH)2��2NH4Cl CaCl2��2NH3����2H2O��
CaCl2��2NH3����2H2O��
��2���������ɵİ����л���ˮ����������ź���ʵ���в���ˮ�IJⶨ����ʯ���Ǹ����������ˮ���������Լ�ʯ�ҵ������Ǹ��ﰱ������ֹ���Ų���ˮ�IJⶨ��
��3����ɫCuO��Ϊ��ɫ��������������ͭ��ͬʱ����һ����ɫ���壬����������Ⱦ����˸������ǵ���������ԭ���غ��֪������ˮ���ɣ���Ӧ�Ļ�ѧ����ʽΪ3CuO+2NH3  3Cu+N2+3H2O����ˮ����ͭ����ˮ�ɰ������Ϊ��ɫ������D�е�ʵ�������ǰ�ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��
3Cu+N2+3H2O����ˮ����ͭ����ˮ�ɰ������Ϊ��ɫ������D�е�ʵ�������ǰ�ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��
��4�������Ǵ̼������壬���Բ��������ŷŵ������У�Ӧ��β������װ�ã�������ͼ��ʾװ�����հ���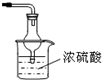 ��
��
��5���ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��
3H2 + N2 2NH3 ��n�L
2NH3 ��n�L
3mol 1mol 2mol 2mol
n(H2) 1mol
���n(H2)�� ��1.5mol
��1.5mol
������������Ũ����1.5mol��2L��0.75mol/L
��������ʱ������H2��ʾ�Ļ�ѧ��Ӧ���ʣ�0.75mol/L��5min��0.15 mol/(L��min)
���㣺���鰱�����Ʊ���������ӡ�����̽����β�������Լ���Ӧ���ʵļ����

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д���ҵβ���е�������ͨ�����ð������շ�����ԭ����NH3��NOx�ڴ��������·�Ӧ�����������ʡ�ijУ�С��ͬѧ��������װ�úͲ���ģ�ҵ�ϵ�������Ĵ������̡�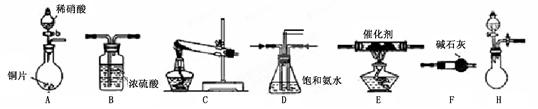
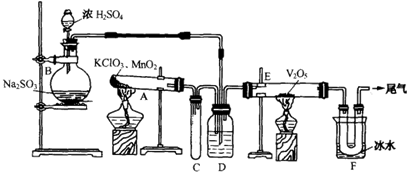
I��̽����ȡNH3�ķ���
��1��������װ���У�H�ܿ��١������ȡNH3��װ������Ҫ���ӵķ�Ӧ�Լ�Ϊ ��
��2��Ϊ̽�����õ�ʵ��Ч�����С��ͬѧ��������Cװ������ȡ�������ڿ���ʵ��������ͬ������£�����±���ʵ�����ݡ�
| �Լ������� | �����Լ� | NH3�����mL�� | |
| a | 6.0 g Ca(OH)2�������� | 5.4 g NH4Cl | 1344 |
| b | 5.4g (NH4)2SO4 | 1364 | |
| c | 6.0 g NaOH�������� | 5.4 g NH4Cl | 1568 |
| d | 5.4g (NH4)2SO4 | 1559 | |
| e | 6.0 g CaO�������� | 5.4 g NH4Cl | 1753 |
| f | 5.4 g (NH4)2SO4 | 1792 | |
�����������ݣ�����Ϊ���ַ�����ȡ������Ч����� ������ţ����Ӹ÷���ѡ���ԭ�Ϸ�������Ч���õĿ���ԭ���� ��
II��ģ��β������
�С��ͬѧѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ý���ʵ�顣
��1���������װ����ѡ������Ϊ�����Ľ��в��䣨��ѡװ�ò����ظ�����
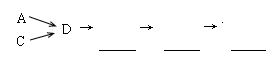
��2��A�з�Ӧ�����ӷ���ʽΪ ��
��3��Dװ�õ������У�ʹ�����Ͼ��ȡ����������ٶȡ� ��
��4��Dװ���е�Һ�廹�ɻ��� ������ţ���
a��H2O b��CCl4 c��ŨH2SO4 d��CuSO4��Һ
��5����С��ͬѧ����Ƶ�ģ��β������װ���л�����һ�����Ե�ȱ���� ��
��ͼ1��ʾ��ij��ȤС��ͬѧ��ͭƬ����ϡ���ᣬ���ֿ�ʼʱ���ݲ������ʷdz�����һ��ʱ����������Լӿ죬��ƿ����Һ��dz��ɫ�����ϼ��Һ���Ϸ���������ɫҲ�ڲ��ϼ����С��ͬѧ��ͨ��ʵ��̽����Ӧ���ʱ仯��ԭ��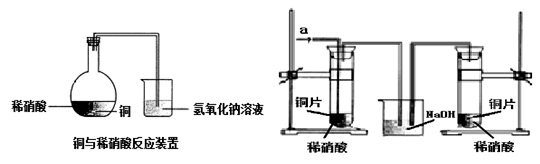
ͼ 1 ͼ 2
��1��ͼ1��ͭ��ϡ���ᷴӦ�����ӷ���ʽΪ ��
�����ӷ���ʽ��ʾNaOH��Һ��������� ��
��2��С��ͬѧ��������¼��貢���ʵ��̽����
��.��ͬѧ��Ϊ�Ƿ�Ӧ���ȵ�����Һ�¶��������£���ɴ�ʵ�黹��Ҫ�������� ��
�ⶨ��Ӧ��������Һ��ͬʱ����¶ȣ�������±���
| ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 35 | 50 | 60 | 70 | 80 |
| �¶�/�� | 25 | 26 | 26 | 26 | 26 | 26 | 26.5 | 27 | 27 | 27 | 27 |
���ʵ��Ŀ�ĺͱ������ݣ���ó��Ľ����� ��
��.��ͬѧ��Ϊ���ɵ�Cu2+�Է�Ӧ�д����ã�Ϊ��֤�˼��裬ȡA��B��֧�Թֱܷ���������ͭƬ��ϡ���ᣬ��ô�����������һ֧�Թ��м��������� ������ţ���
A.����ͭ���� B.����ͭ��Һ C.����ͭ���� D.����ͭ��Һ
Ȼ��Ա���֧�Թܵķ�Ӧ���������������ͬ����˵ó����ۣ�Cu2+�����Ƿ�Ӧ�Ĵ�����
��. ��ͬѧ���������ƲⷴӦ�����л������� ���ɣ�������Ϊ�����ʶԷ�Ӧ�д����ã���ͼ2��ʾ��ʵ���б�ͬѧ��a��ͨ������ʺ�������в��������������Կ����ҹܡ�С��ͬѧ�ó������ۣ��������ʶ�ͭ��ϡ����ķ�Ӧ�д����á�
��3��ʵ����������Թ�����Һ����ɫ����������ɫ������ͬѧ��Ϊ�Ǹ���Һ������ͭ�����������ϸ����£���һ����ͬѧ��Ϊ�Ǹ���Һ���ܽ���ͨ������ʡ��������һ��ʵ�鷽����֤�����ּ�����ȷ��(д��ʵ�������ʵ������ͽ���) ��
��֪ij����X�ܷ�������ת����
�����й�����ת����ϵ�����ʼ��䷴Ӧ�������������
| A����XΪN2��NH3��������������AΪ���� |
| B����XΪS ��H2S����ȫȼ�գ�����AΪ���� |
| C����XΪ�ǽ������ʻ�ǽ����⻯���A��һ���������ͭ��Ӧ����Y |
| D����Ӧ�ٺ͢�һ��Ϊ������ԭ��Ӧ����Ӧ��һ��Ϊ��������ԭ��Ӧ |


 ��Һ��������________________��
��Һ��������________________��