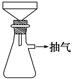
��Ŀ����
��������淋Ļ�ѧʽΪ��NH4��2SO4?FeSO4?6H2O����Ʒ��ΪĪ���Σ�������������������立�Ӧ���ɣ�һ�������������ڿ������ױ����������γ�Ī���κ�ͱȽ��ȶ��ˣ������ε��ܽ�ȣ���λΪg/100gˮ�����±���| �¶�/�� | 10 | 20 | 30 |
| ��NH4��2SO4 | 73.0 | 75.4 | 78.0 |
| FeSO4?7H2O | 20.0 | 26.5 | 32.9 |
| ��NH4��2SO4?FeSO4 | 17.2 | 21.6 | 28.1 |
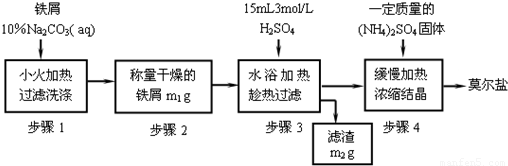
�Իش��������⣺
��1������1�м���10% Na2CO3��Һ����Ҫ������______����Ӧ����м������Ϊ��______��
��2������3��Ҫ���ȹ��ˣ�ԭ����______��
��3���Ӳ���4��Ī���Σ�������еIJ���������______�������ľ��峣��______ϴ�ӣ�
��4����Ī���εı�����Һ����ˮ20�ˣ����¶ȴ�30�潵��10�棬�������Ī���ε�������______��ѡ���ţ���
A.2.18g B������2.18g C��С�� 2.18g D����ȷ��
��������ȡ����Ϊ1.96g��Ī�����Ƴ���Һ����δ֪Ũ�ȵ�����KMnO4��Һ���еζ���
��1����֪MnO4-����ԭΪMn2+����д���õζ���Ӧ�����ӷ���ʽ______��
��2���жϸ÷�Ӧ����ζ��յ������Ϊ______��
��3�����赽��ζ��յ�ʱ����ȥVmL����KMnO4��Һ���������KMnO4��Һ��Ũ��Ϊ______mol/L��
���𰸡���������һ����1��̼����ˮ�������ԣ���ԭ�������ɵ�Fe3+����2��FeSO4���¶ȵ�ʱ�ܽ�Ƚ�С��
��3��Ũ���ᾧ����Ҫ���ˡ�ϴ�ӣ��¶ȵ�ʱ����������淋��ܽ��С��
��4����NH4��2SO4?FeSO4��30�Ⱥ�10�ȵ��ܽ�ȷֱ�Ϊ��28.1��17.2g��
��������1��MnO4-����������������Ϊ���������ӣ�����ԭΪMn2+��
��2��������ر�������ɫ���ζ����������Dz���Ҫָʾ���ģ�
��3�������������Ӻ��������Ӧ��ʵ�ʣ������ҵ�����������������֮������Ĺ�ϵ������ԭ���غ�����ҵ��������Ӻ��������֮�����Ĺ�ϵ���������м��㣮
����⣺��һ����1��̼����ˮ�������ԣ���֬�ڼ�����������ˮ�⣻��ԭ�������ɵ�Fe3+�����ٲ����е�Fe3+���ʣ�
�ʴ�Ϊ������м��������ۣ���ԭ�������ɵ�Fe3+����֤Fe2+�ȶ��������ش��ڣ����ٲ����е�Fe3+���ʣ�
��2����������ȹ��˾ͻ���FeSO4?7H2O�������ʴ�Ϊ��FeSO4���¶ȵ�ʱ�ܽ�Ƚ�С����������ȹ��˾ͻ���FeSO4?7H2O������
��3��Ũ���ᾧ����Ҫ���ˡ�ϴ�ӣ��������������ˮ�Ҵ����ܵ��ܽ��С���¶ȵ�ʱ����������淋��ܽ��С�����ñ�ˮϴ�ӣ��ʴ�Ϊ�����ˡ�ϴ�ӣ���ˮ�ƾ����ˮ��
��4����NH4��2SO4?FeSO4��30�Ⱥ�10�ȵ��ܽ�ȷֱ�Ϊ��28.1��17.2g�������ܼ�Ϊ100gˮ����ȴ����10.9g����ˮ20������2.18g����������淋Ļ�ѧʽΪ��NH4��2SO4?FeSO4?6H2O���нᾧˮ����������������2.18g���ʴ�Ϊ��B��
��������1����Ӧ�����ӷ���ʽ5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O���ʴ�Ϊ��5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
��2��������ر�������ɫ���ζ��������Ӳ���Ҫָʾ�������μ����һ����Һ����Һ����Ϻ�ɫ��30S�ڲ���ɫ��˵���ﵽ�ζ��յ㣬�ʴ�Ϊ���������һ��KMnO4��Һ�Ϻ�ɫ���ʣ��Ұ�����ڲ���ɫ��
��3��1.96g��������茶�������ʵ���n=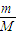 =
=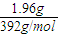 =0.005mol������ԭ���غ����������ӵ����ʵ���Ϊ0.005mol����Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O����5Fe2+��MnO4-�����Ը�����ص����ʵ���Ϊ0.001mol����c=
=0.005mol������ԭ���غ����������ӵ����ʵ���Ϊ0.005mol����Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O����5Fe2+��MnO4-�����Ը�����ص����ʵ���Ϊ0.001mol����c=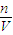 =
=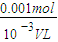 =
=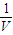 mol/l���ʴ�Ϊ��
mol/l���ʴ�Ϊ��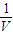 ��
��
���������⿼���Ʊ�ʵ�鷽������ƣ��ۺ��Խ�ǿ��ע�⣨4���о�������ʱ���ᾧˮΪ�����״��㣬�ѶȽϴ�
��3��Ũ���ᾧ����Ҫ���ˡ�ϴ�ӣ��¶ȵ�ʱ����������淋��ܽ��С��
��4����NH4��2SO4?FeSO4��30�Ⱥ�10�ȵ��ܽ�ȷֱ�Ϊ��28.1��17.2g��
��������1��MnO4-����������������Ϊ���������ӣ�����ԭΪMn2+��
��2��������ر�������ɫ���ζ����������Dz���Ҫָʾ���ģ�
��3�������������Ӻ��������Ӧ��ʵ�ʣ������ҵ�����������������֮������Ĺ�ϵ������ԭ���غ�����ҵ��������Ӻ��������֮�����Ĺ�ϵ���������м��㣮
����⣺��һ����1��̼����ˮ�������ԣ���֬�ڼ�����������ˮ�⣻��ԭ�������ɵ�Fe3+�����ٲ����е�Fe3+���ʣ�
�ʴ�Ϊ������м��������ۣ���ԭ�������ɵ�Fe3+����֤Fe2+�ȶ��������ش��ڣ����ٲ����е�Fe3+���ʣ�
��2����������ȹ��˾ͻ���FeSO4?7H2O�������ʴ�Ϊ��FeSO4���¶ȵ�ʱ�ܽ�Ƚ�С����������ȹ��˾ͻ���FeSO4?7H2O������
��3��Ũ���ᾧ����Ҫ���ˡ�ϴ�ӣ��������������ˮ�Ҵ����ܵ��ܽ��С���¶ȵ�ʱ����������淋��ܽ��С�����ñ�ˮϴ�ӣ��ʴ�Ϊ�����ˡ�ϴ�ӣ���ˮ�ƾ����ˮ��
��4����NH4��2SO4?FeSO4��30�Ⱥ�10�ȵ��ܽ�ȷֱ�Ϊ��28.1��17.2g�������ܼ�Ϊ100gˮ����ȴ����10.9g����ˮ20������2.18g����������淋Ļ�ѧʽΪ��NH4��2SO4?FeSO4?6H2O���нᾧˮ����������������2.18g���ʴ�Ϊ��B��
��������1����Ӧ�����ӷ���ʽ5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O���ʴ�Ϊ��5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
��2��������ر�������ɫ���ζ��������Ӳ���Ҫָʾ�������μ����һ����Һ����Һ����Ϻ�ɫ��30S�ڲ���ɫ��˵���ﵽ�ζ��յ㣬�ʴ�Ϊ���������һ��KMnO4��Һ�Ϻ�ɫ���ʣ��Ұ�����ڲ���ɫ��
��3��1.96g��������茶�������ʵ���n=
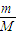 =
=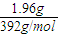 =0.005mol������ԭ���غ����������ӵ����ʵ���Ϊ0.005mol����Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O����5Fe2+��MnO4-�����Ը�����ص����ʵ���Ϊ0.001mol����c=
=0.005mol������ԭ���غ����������ӵ����ʵ���Ϊ0.005mol����Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O����5Fe2+��MnO4-�����Ը�����ص����ʵ���Ϊ0.001mol����c=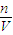 =
=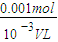 =
=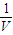 mol/l���ʴ�Ϊ��
mol/l���ʴ�Ϊ��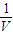 ��
�����������⿼���Ʊ�ʵ�鷽������ƣ��ۺ��Խ�ǿ��ע�⣨4���о�������ʱ���ᾧˮΪ�����״��㣬�ѶȽϴ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��������淋Ļ�ѧʽΪ��NH4��2SO4?FeSO4?6H2O����dz��ɫ���壬��Ʒ��ΪĪ���Σ�������ˮ���������Ҵ���
��������ʵ�鱨�棺
��ʵ��ԭ����
��ʵ����Ʒ��
10%Na2CO3��Һ��Feм��3mol/LH2SO4����NH4��2SO4���塢����ˮ����ˮ�Ҵ�����ƿ�������ƾ��ơ����������ձ�������̨��©����������ƽ����Ͳ����ֽ��
��ʵ�鲽�衿
��1��Feм�Ĵ����ͳ���
��ȡ3g��м��������ƿ����l0%Na2CO3��Һ����ȥFeм��������ۣ���ʣ��ļ�Һ������������ˮ��Fe��ϴ�ɾ���������������m1g��
��2��FeSO4���Ʊ�
�������õ�Feм������ƿ�У�����l5mL 3mol/LH2SO4������10min�����������ɣ����������ע�ⰲȫ���� ������������ˮϴ����ƿ����ֽ������Һ��ϴ��Һһ��ת�����������У�����ֽ�ϵĹ�������Ƶ�����Ϊm2g��
��a���IJ����� ��
����ȡFeSO4ʱ�������FeΪʲôҪ�������������ӷ���ʽ��ʾ��
��3����NH4��2SO4?FeSO4?6H2O���Ʊ�
����FeSO4�����ʵ��������㲢��ȡ��NH4��2SO4���壬�������������ʵ����������У��������ȣ�Ũ����������ֽᾧ��ĤΪֹ�� ���õ���������淋ľ��壮���˺�����ˮ�Ҵ�ϴ�Ӿ��壬��ȥ������ˮ�֣������õ��ľ�������Ϊm3g��
��b���IJ����� �����Ʊ�Ī���ξ���ʱ��Ϊʲô���ܽ���Һ�������ɣ�
��4����Ʒ����
��Fe3+�ķ�������0.5g��Ʒ����25mL��ɫ���У���ˮ�ܽ���1mL 25%��KSCN��Һ��������ˮ��25mL�̶ȣ�ҡ�ȣ����ʦ�������Աȣ����۲�Ʒ������д���ù�����������Ӧ�����ӷ���ʽ��
��ʵ���¼��
| �¶�/�� �ܽ��/g �� |
0 | 10 | 20 | 30 | 40 | 50 | 60 |
| ��NH4��2SO4 | 70.6 | 73.0 | 75.4 | 78.0 | 81.0 | - | 88.0 |
| FeSO4?7H2O | 15.7 | 20.5 | 26.5 | 32.9 | 40.2 | 48.6 | - |
| ��NH4��SO4FeSO4?6H2O | 12.5 | 17.2 | 21.6 | 28.1 | 33.0 | 40.0 | - |
��ʵ��ԭ����
��ʵ����Ʒ��
10%Na2CO3��Һ��Feм��3mol/LH2SO4����NH4��2SO4���塢����ˮ����ˮ�Ҵ�����ƿ�������ƾ��ơ����������ձ�������̨��©����������ƽ����Ͳ����ֽ��
��ʵ�鲽�衿
��1��Feм�Ĵ����ͳ���
��ȡ3g��м��������ƿ����l0%Na2CO3��Һ����ȥFeм��������ۣ���ʣ��ļ�Һ������������ˮ��Fe��ϴ�ɾ���������������m1g��
��2��FeSO4���Ʊ�
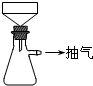
�������õ�Feм������ƿ�У�����l5mL 3mol/LH2SO4������10min�����������ɣ����������ע�ⰲȫ����
��a���IJ�����
����ȡFeSO4ʱ�������FeΪʲôҪ�������������ӷ���ʽ��ʾ��
��3����NH4��2SO4?FeSO4?6H2O���Ʊ�
����FeSO4�����ʵ��������㲢��ȡ��NH4��2SO4���壬�������������ʵ����������У��������ȣ�Ũ����������ֽᾧ��ĤΪֹ��
��b���IJ�����
��4����Ʒ����
��Fe3+�ķ�������0.5g��Ʒ����25mL��ɫ���У���ˮ�ܽ���1mL 25%��KSCN��Һ��������ˮ��25mL�̶ȣ�ҡ�ȣ����ʦ�������Աȣ����۲�Ʒ������д���ù�����������Ӧ�����ӷ���ʽ��
��ʵ���¼��
| ��ʼ��������m1/g | ��Ӧ����������m2/g | Ī���ε����� | ���� | |
| ���۲���/g | ʵ�ʲ���m3/g | |||
| 5.0 | 2.2 | c | 14.7 | d |

 ��ʵ�黯ѧ��
��ʵ�黯ѧ��