��Ŀ����
�����йػ�ѧ����ı�����ȷ���ǣ�NA��ʾ����٤���������� ��A��̼�������������������������Һ��ϣ�HCO3-+OH-=CO32-+H2O
B�������е�SO2��Ʈ���������������»Ჿ��ת��ΪSO3��ʹ������������ӣ�������Ⱦ��Ϊ����
C�����³�ѹ�£�2.24L���������������������Һ��ȫ��Ӧת�Ƶĵ�����Ϊһ��Ϊ0.1NA
D�������£��������ơ���������Һ��ϳ����Ե���Һ�У�c��Na+����c��Cl-��=c��CH3COO-��
���𰸡�������A�������������������ɰ��������жϣ�
B����������γɷ����жϣ�
C����������Ħ�������Ӧ�����������жϣ�
D��������Һ�е���غ�������㣻
����⣺A��̼�������������������������Һ��ϵ����ӷ���ʽӦΪ��NH4++HCO3-+2OH-=CO32-+2H2O+NH3������A����
B�������е�SO2�ŷŵ������У���Ʈ���������������»Ჿ��ת��ΪSO3�������γ����ᣬ�γ�Σ���Դ�����꣬��B��ȷ��
C��2.24L�������³�ѹ�²�һ����0.1mol������Ӧʱ����ת�Ʋ�һ����0.1NA����C����
D�������ơ���������Һ��ϳ�����ʱ�ĵ���غ�Ϊ��[Na+]+[H+]=[Cl-]+[CH3COO-]+[OH-]��Һ������[H+]=[OH-]�����Եõ���[Na+]=[Cl-]+[CH3COO-]����c��Cl-����һ������c��CH3COO-������D����
��ѡB��
���������⿼�����й��������ӷ���ʽ����д��������γɣ�����Ħ�������Ӧ����������Һ�еĵ���غ㣮
B����������γɷ����жϣ�
C����������Ħ�������Ӧ�����������жϣ�
D��������Һ�е���غ�������㣻
����⣺A��̼�������������������������Һ��ϵ����ӷ���ʽӦΪ��NH4++HCO3-+2OH-=CO32-+2H2O+NH3������A����
B�������е�SO2�ŷŵ������У���Ʈ���������������»Ჿ��ת��ΪSO3�������γ����ᣬ�γ�Σ���Դ�����꣬��B��ȷ��
C��2.24L�������³�ѹ�²�һ����0.1mol������Ӧʱ����ת�Ʋ�һ����0.1NA����C����
D�������ơ���������Һ��ϳ�����ʱ�ĵ���غ�Ϊ��[Na+]+[H+]=[Cl-]+[CH3COO-]+[OH-]��Һ������[H+]=[OH-]�����Եõ���[Na+]=[Cl-]+[CH3COO-]����c��Cl-����һ������c��CH3COO-������D����
��ѡB��
���������⿼�����й��������ӷ���ʽ����д��������γɣ�����Ħ�������Ӧ����������Һ�еĵ���غ㣮

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
�����Ŀ
��8�֣��±���Ԫ�����ڱ���ǰ������ȥ�������Ϸ��Ŀհ��������϶��ɣ��������ߴ�Ϊ��A����A������Ӵ���������Ӧ�Ļ�ѧ�������Żش��������⣺
| a | | | | | | | |
| b | | | c | d | e | f | |
| g | | h | | | | | |

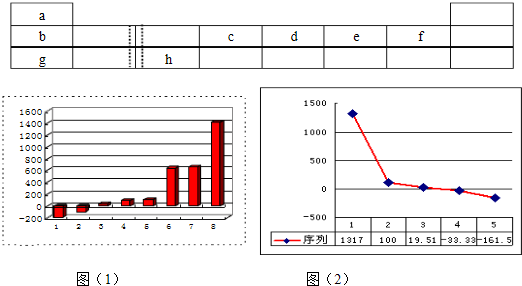
��1����ͼ��1���DZ�ʾ��������8��Ԫ�ص��ʵ��۵㣨�棩����ͼ����֪���Ρ�1������Ar�����������Ρ�8���������� ���壨�������ͣ���
��2��b��c��d��e��f���⻯��ķе㣨�棩ֱ������ͼ��2�������С�5�����⻯���������
�����С�2�����⻯��ĽṹʽΪ ��
��3��eԪ����fԪ����ȣ��縺��f����e�����б�������֤����һ��ʵ���� ����ѡ����ţ�
A��������f���ʵ���ɫ��e���ʵ���ɫ��
B��f������e���⻯����ҷ�Ӧ������e�ĵ���
C��f��e�γɵĻ�������eԪ�س�����̬
D���Ƚ���Ԫ�صĵ�������������ʱ�õ��ӵ���Ŀ
��4����ѧ��֤ʵ���Ȼ������ڹ��ۻ��������ʽΪAl2Cl6����ÿ��Ԫ�ؾ�����8���ӵĽṹ����д������Ϊ��ȷ��Al2Cl6�ṹ��_______________��
��5������������һ����Ҫ�������ըҩ�����Ի������о�����Ԫ�زⶨ����Խ��Խ�������ǵ����ӣ��ɵ�������(Sodium azida)NaN3�ȷֽ�ɵù��״�N2��2NaN3(s)��2Na(l)+3N2(g)���й�˵����ȷ����_____________(ѡ�����)

A���ƾ����ṹ��ͼ���ƾ�����ÿ����ԭ�ӵ���λ��Ϊ6
B���ƾ����ṹ��ͼ�������з�̯2����ԭ��
C�����ĵ縺�Դ�����
D��Na+�İ뾶С��N3-�İ뾶