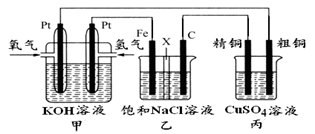
��Ŀ����
��ҵ�ϵ���Ƽ�ļ������������ӽ���Ĥ������Ҫԭ���DZ���ʳ��ˮ����ͼΪ�����ӽ���Ĥ�����ԭ��ʾ��ͼ��
��ش��������⣺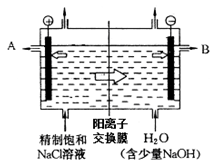
��1��A��Ϊ���۵�________����B���IJ��Ϲ�ҵ�ϳ����õ��������г����Ľ��������д�����ģ�����Ҫԭ���� �����������ʴ�������ⸯʴ����
��2�������в��������ӽ���Ĥ�ѵ��۸����������Һ������ң����������ӽ���Ĥ��ֻ������Һ�е�__________ͨ������д�������ı�ţ���
��H2����Cl2����H+����Cl-����Na+����OH-��
��3�����������������������ӽ���Ĥ���������ӽ���Ĥ����֪��3Cl2 +6OH-==5Cl-+ClO3-+3H2O��������˵����ȷ������� ��
| A��������ʱ���Ҳ���Һ�к���ClO3�� |
B�������ڷ�����Ӧ���ܻ�ѧ����ʽΪ��NaCl + 3H2O  NaClO3 + 3H2�� NaClO3 + 3H2�� |
| C���������ӽ���Ĥ�����������缫�����ĵ缫��Ӧ��ԭ��һ�� |
| D���������ӽ���Ĥ�������ӵĶ����ƶ�������ԭ���෴ |

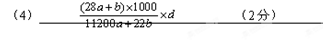
���������������ͼ��֪�������Ӵ���������ұߣ��ڵ����У��������Һ�е������������������������������������A��������B�������������л���ڴ�����������ˮ��������˸ý���������ĵ���Ҫԭ���Ƿ�����������ʴ�������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ����ˣ�2�������ӽ���Ĥֻ�����ۢ�ͨ�������������Ӻ������ӻ����������ƶ�������ClO3�������ƶ����پݵ������������ӵķŵ�˳��֪������������������ʧȥ�����������������������������ӵõ�����������������˵����ڷ�����Ӧ���ܻ�ѧ����ʽΪ��NaCl + 3H2O  NaClO3 + 3H2�������ӽ���Ĥ���ܸı�缫��Ӧʽ��Ҳ���ܸı��������������ƶ����������������ƶ�����ʵ�����Ϊ��3��ΪABCѡ���4�����ù�ʽŨ�ȵ���1000�����ܶȳ������������ٳ���Ħ��������ó���
NaClO3 + 3H2�������ӽ���Ĥ���ܸı�缫��Ӧʽ��Ҳ���ܸı��������������ƶ����������������ƶ�����ʵ�����Ϊ��3��ΪABCѡ���4�����ù�ʽŨ�ȵ���1000�����ܶȳ������������ٳ���Ħ��������ó���
���㣺������ؼ����ʵ���Ũ�ȵ����֪ʶ��

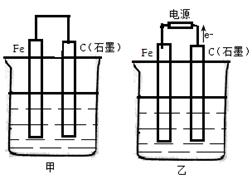
����װ���У�Fe��ʴ�ɿ쵽����˳��Ϊ�� ��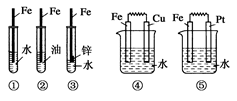
| A���ݢܢۢڢ� |
| B���ݢܢ٢ڢ� |
| C���ݢۢܢ٢� |
| D���٢ڢܢۢ� |
����������ȷ����
| A���ڵ��ص�������ԭ��صĸ����϶�����������Ӧ |
| B���Ʋ������������ȶ�п�������ʴ |
| C���ö��Ե缫���KOH��Һ��������������������ʵ���֮��Ϊ1��2 |
| D���ö��Ե缫��ⱥ��NaCl��Һ������1 mol����ת�ƣ�������1 molNaOH |
��14�֣�Ϊ�������÷Ϸ�����������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ���Ҫ�������£�
���ֺ���������ˮ�е��ܽ������£�
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
�Ź�ҵ��V2O5ұ���������������ȼ�����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��ͼ����ʾ��Һ�к�������Ҫ�ɷ�Ϊ ��д��ѧʽ����
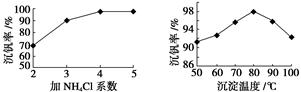
�Ǹù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ���ò���Ӧ�����ӷ���ʽ �������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ����NH4Cl������������Һ��V2O5�������ȣ����¶ȡ�������ͼ�ж���ѿ����Ȼ��ϵ�����¶�Ϊ �� ��
���������ữ��H2C2O4��Һ�ζ���VO2��2SO4��Һ���Բⶨ��Ӧ�ں���Һ�к���������ɷ�Ӧ�����ӷ���ʽΪ��VO2+����H2C2O4����_____����VO2+����CO2������H2O��
��ȫ��Һ����صĵ������ҺΪVOSO4��Һ����صĹ���ԭ��ΪVO2+��V2+��2H+
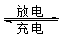 VO2+��H2O��V3+����س��ʱ�����ĵ缫��ӦʽΪ ��
VO2+��H2O��V3+����س��ʱ�����ĵ缫��ӦʽΪ �� 

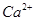 ��
�� ��
�� ���ӣ��������г�������˳���ǣ�����ţ�_____________ ��
���ӣ��������г�������˳���ǣ�����ţ�_____________ ��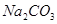 B��
B�� 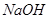 C��
C�� 
 �������Գ�ȥ��������___________________��
�������Գ�ȥ��������___________________�� ��Һ�����ӷ���ʽ��_____________________��
��Һ�����ӷ���ʽ��_____________________��
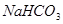 ������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����壬�仯ѧʽ�� _____________________ ��
������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����壬�仯ѧʽ�� _____________________ ��