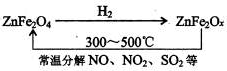
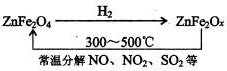
��Ŀ����
��12�֣�����������±���������������������4FeS2��11O2 8SO2��2Fe2O3
8SO2��2Fe2O3
�������O2��N2����������ֱ�Ϊ20%��80%����������и��⣺
��1��1 mol FeS2��ȫ������������(�����Ǹ���������ʧ������ͬ)��Ҫ�����������״����Ϊ L��
��2��55 L����(��״��)������FeS2��ȫ��Ӧ�����������Ϊ(��״��) L��
��3���ڹ�ҵ����H2SO4�У�Ϊ���SO2��ת���ʣ�����Ӵ��ҵ�SO2��O2�����������Ϊ1��1����ô�ڹ�ҵ����������1mol FeS2��������������(��״����Ϊ___________L������Ӵ��ҵĻ��������SO2���������Ϊ____________��
(1)308 (2)52 (3)532 (4)8.70%
����

��ϰ��ϵ�д�
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ