��Ŀ����
��֪ij�¶��£����ڿ��淴ӦA(g)��B(g) 2C(g)���ڸ��¶��£����ݻ�Ϊ2L�ĺ����ܱ������м���2 mol A��2 mol B����1minʱ�ﵽƽ�⣬���������C�����������x(C)����ʱ��(t)�仯��������ͼ��ʾ����2minʱ����������м���2 mol C����3minʱ��Ӧ���´ﵽƽ����4min����
2C(g)���ڸ��¶��£����ݻ�Ϊ2L�ĺ����ܱ������м���2 mol A��2 mol B����1minʱ�ﵽƽ�⣬���������C�����������x(C)����ʱ��(t)�仯��������ͼ��ʾ����2minʱ����������м���2 mol C����3minʱ��Ӧ���´ﵽƽ����4min����
��1���÷�Ӧ��ǰ1min��ƽ��ʱA��ת���ʡ�
��2�����¶��µ�ƽ�ⳣ����
��3��������ͼ�н�0��4min�ı仯����ͼ��������������ע��ͼ��������Ķ�Ӧ��ֵ����
 2C(g)���ڸ��¶��£����ݻ�Ϊ2L�ĺ����ܱ������м���2 mol A��2 mol B����1minʱ�ﵽƽ�⣬���������C�����������x(C)����ʱ��(t)�仯��������ͼ��ʾ����2minʱ����������м���2 mol C����3minʱ��Ӧ���´ﵽƽ����4min����
2C(g)���ڸ��¶��£����ݻ�Ϊ2L�ĺ����ܱ������м���2 mol A��2 mol B����1minʱ�ﵽƽ�⣬���������C�����������x(C)����ʱ��(t)�仯��������ͼ��ʾ����2minʱ����������м���2 mol C����3minʱ��Ӧ���´ﵽƽ����4min������1���÷�Ӧ��ǰ1min��ƽ��ʱA��ת���ʡ�
��2�����¶��µ�ƽ�ⳣ����
��3��������ͼ�н�0��4min�ı仯����ͼ��������������ע��ͼ��������Ķ�Ӧ��ֵ����

��1��50%
��2��4
��3��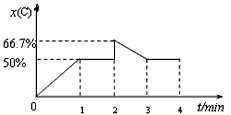
��2��4
��3��


��ϰ��ϵ�д�
�����Ŀ
 ��2011?�����ģ����ش��й�п���仯��������⣮
��2011?�����ģ����ش��й�п���仯��������⣮ Zn2+��aq��+2OH-��aq������Ksp[Zn��OH��2]��Ksp��ZnS���������S2-��Zn2+��������˸����ܣ����ܶȻ���С����ZnS��ʹZn2+Ũ�ȼ�С���ܽ�ƽ����������ƶ�ֱ��ȫ��ת��ΪZnS
Zn2+��aq��+2OH-��aq������Ksp[Zn��OH��2]��Ksp��ZnS���������S2-��Zn2+��������˸����ܣ����ܶȻ���С����ZnS��ʹZn2+Ũ�ȼ�С���ܽ�ƽ����������ƶ�ֱ��ȫ��ת��ΪZnS

 B��������ε���̼������Һ��ǡ����ȫ��Ӧʱ��Һ������
B��������ε���̼������Һ��ǡ����ȫ��Ӧʱ��Һ������