��Ŀ����
�±���Ԫ�����ڱ��ж�����Ԫ�ص�һ���֣�����������ĸ�ֱ����һ��Ԫ�أ�| A | B | ||||||
| D | E | F | |||||
| C | G | H |
��2��D���⻯���G���⻯���ȶ�����ԭ����______��
��3����һ�������£�A��E���γ�һ�ּ�������ˮ����̬����������ʽΪ______�������ʵ�ˮ��Һ�е����̪����Һ��______ ɫ��
��4����Ԫ�ط��ű�ʾ��D��E��F��G��ԭ�Ӱ뾶�ɴ�С˳��______��
��5�������ۡ��ߺ����˷ɴ�����Ҫ��һ�ֻ����������պ���Ա������CO2��ͬʱΪ����Ա�ṩ��Ҫ�����壬�����û�ѧ����ʽ��ʾ����ԭ����______��
�ɴ�����Ҫ����һ���ʺϺ���Ա������˹�̬��������Ӧ���������г���һ��ϡ�����壬�����廯ѧʽΪ______��
���𰸡���������1������Ԫ�������ڱ��еķֲ�֪ʶ���ش�
��2��ԭ�ӵĵõ�������Խǿ���γɵ��⻯����ȶ���Խǿ��
��3��������ԭ�Ӻ͵�ԭ��֮��ͨ�����ۼ��γɵĹ��ۻ��������ˮ��Һ�Լ��ԣ���ʹ��̪��Һ���ɫ��
��4�����Ӳ�Խ��뾶Խ���Ӳ�һ��ʱ���˵����Խ��뾶ԽС��
��5���������ƺͶ�����̼��Ӧ�����������������ƿ����������Ĺ�������
����⣺����Ԫ�������ڱ��еķֲ�֪ʶ������ȷ��A��H��C��Na��B��He��D��C��E��N��F��O��G��Si��H��Cl��
��1��ClԪ�������ڱ��е�λ��Ϊ�������ڢ�A�壬�ʴ�Ϊ���������ڢ�A�壻
��2��ͬ����Ԫ�ص�ԭ�Ӵ��ϵ���ԭ�ӵĵõ�������Խ��Խ��������Cԭ�ӵõ���������Siǿ��ԭ�ӵĵõ�������Խǿ���γɵ��⻯����ȶ���Խǿ���ʼ����ȶ���ǿ�ڹ��飬�ʴ�Ϊ��Dԭ�ӵõ���������Gǿ��
��3��������ԭ�Ӻ͵�ԭ��֮��ͨ�����ۼ��γɵĹ��ۻ��������ʽΪ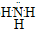 ��������������ˮ������ˮ��Һ��ˮ�Լ��ԣ���ʹ��̪��Һ���ɫ���ʴ�Ϊ��
��������������ˮ������ˮ��Һ��ˮ�Լ��ԣ���ʹ��̪��Һ���ɫ���ʴ�Ϊ��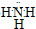 ���죻
���죻
��4��Si�ĵ��Ӳ��뾶���C��N��O���Ӳ�һ�����˵����Խ��뾶ԽС����C��N��O���ʴ�Ϊ��Si��C��N��O��
��5���������ƺͶ�����̼��Ӧ����������Ϊϡ������������һ���ʺ��Ա������˹���̬�����������ڷɴ��мӵ������������ƿ����Ļ�����
�ʴ�Ϊ��2Na2O2+2CO2�T2Na2CO3+O2��N2��
���������⿼��ѧ��Ԫ�����ڱ��Ľṹ��Ԫ��������֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���
��2��ԭ�ӵĵõ�������Խǿ���γɵ��⻯����ȶ���Խǿ��
��3��������ԭ�Ӻ͵�ԭ��֮��ͨ�����ۼ��γɵĹ��ۻ��������ˮ��Һ�Լ��ԣ���ʹ��̪��Һ���ɫ��
��4�����Ӳ�Խ��뾶Խ���Ӳ�һ��ʱ���˵����Խ��뾶ԽС��
��5���������ƺͶ�����̼��Ӧ�����������������ƿ����������Ĺ�������
����⣺����Ԫ�������ڱ��еķֲ�֪ʶ������ȷ��A��H��C��Na��B��He��D��C��E��N��F��O��G��Si��H��Cl��
��1��ClԪ�������ڱ��е�λ��Ϊ�������ڢ�A�壬�ʴ�Ϊ���������ڢ�A�壻
��2��ͬ����Ԫ�ص�ԭ�Ӵ��ϵ���ԭ�ӵĵõ�������Խ��Խ��������Cԭ�ӵõ���������Siǿ��ԭ�ӵĵõ�������Խǿ���γɵ��⻯����ȶ���Խǿ���ʼ����ȶ���ǿ�ڹ��飬�ʴ�Ϊ��Dԭ�ӵõ���������Gǿ��
��3��������ԭ�Ӻ͵�ԭ��֮��ͨ�����ۼ��γɵĹ��ۻ��������ʽΪ
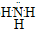 ��������������ˮ������ˮ��Һ��ˮ�Լ��ԣ���ʹ��̪��Һ���ɫ���ʴ�Ϊ��
��������������ˮ������ˮ��Һ��ˮ�Լ��ԣ���ʹ��̪��Һ���ɫ���ʴ�Ϊ��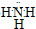 ���죻
���죻��4��Si�ĵ��Ӳ��뾶���C��N��O���Ӳ�һ�����˵����Խ��뾶ԽС����C��N��O���ʴ�Ϊ��Si��C��N��O��
��5���������ƺͶ�����̼��Ӧ����������Ϊϡ������������һ���ʺ��Ա������˹���̬�����������ڷɴ��мӵ������������ƿ����Ļ�����
�ʴ�Ϊ��2Na2O2+2CO2�T2Na2CO3+O2��N2��
���������⿼��ѧ��Ԫ�����ڱ��Ľṹ��Ԫ��������֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
�����Ŀ
 �±���Ԫ�����ڱ��ж�����Ԫ�ز��֣�������ĸ�ֱ����һ��Ԫ�أ�
�±���Ԫ�����ڱ��ж�����Ԫ�ز��֣�������ĸ�ֱ����һ��Ԫ�أ�
