��Ŀ����
 ����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У�
����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У���1��CrO3����ǿ�����ԣ������л����ƾ���ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ�������[Cr2��SO4��3]����÷�Ӧ�Ļ�ѧ����ʽΪ��
��2��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯��ͼ��ʾ��
��A ��ʱʣ�����ijɷ���
�ڴӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ
��3��CrO3�� K2Cr2O7��������ˮ�����ǹ�ҵ����ɸ���Ⱦ����Ҫԭ������������֮һ�ǽ���+6�� Cr �ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2+��Cr2O72-������Ӧ�����ɵ�Fe3+��Cr3+����������OH-������� Fe��OH��3 ��Cr��OH��3������ȥ[��֪ KspFe��OH��3=4.0��10-38��KspCr��OH��3=6.0��10-31]��
�ٵ������� NaCl ��������
����֪�������Һ��c��Fe3+��Ϊ2.0��10-13 mol?L-1������Һ��c��Cr3+��Ϊ
��������1�����ݻ��ϼ�������������Լ�ԭ���غ������
��2���ٸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬�����ԭ�Ӻ�ԭ�ӵĸ����ȼ��ɣ�
�������B�Ĺ���ijɷ֣�������ԭ���غ�д������ʽ��
��3����NaClΪ����ʣ�����������NaCl����ǿ��Һ�ĵ���������
���ȸ���KspFe��OH��3��c��Fe3+�����c��OH-����Ȼ���ٸ���c��OH-����KspCr��OH��3���c��Cr3+����
��2���ٸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬�����ԭ�Ӻ�ԭ�ӵĸ����ȼ��ɣ�
�������B�Ĺ���ijɷ֣�������ԭ���غ�д������ʽ��
��3����NaClΪ����ʣ�����������NaCl����ǿ��Һ�ĵ���������
���ȸ���KspFe��OH��3��c��Fe3+�����c��OH-����Ȼ���ٸ���c��OH-����KspCr��OH��3���c��Cr3+����
����⣺��1��CrO3����ǿ�����ԣ������л����ƾ���ʱ���Ҵ������������ᣬ̼��ƽ�����ϼ۴�-2�����ߵ�0��1���Ҵ����ϼ۱仯4��CrO3����ԭ����ɫ�������[Cr2��SO4��3]�����Ļ��ϼ۴�+6�۽��͵�+3�ۣ�1��CrO3���ϼ۱仯3�����ߵ���С��������12���ٸ���ԭ���غ��4CrO3+3CH3CH2OH+12H+�T4Cr3++3CH3COOH+9H2O��
�ʴ�Ϊ��4CrO3+3CH3CH2OH+12H+�T4Cr3++3CH3COOH+9H2O��
��2������CrO3������Ϊ100g����CrO3�и�Ԫ�ص�����Ϊ��100g��
=52g��A��ʱ���������Ϊ��100g��94.67%=94.67g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ42.67g�����ߵĸ�����Ϊ
��
=3��8������A��ʱʣ�����ijɷ���Cr3O8���ʴ�Ϊ��Cr3O8��
��B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ
��
=2��3������B��ʱʣ�����ijɷ���Cr2O3�����Լ��ȵ� 750K ʱ�ɷ���Cr2O3����Ӧ����ʽΪ��4CrO3
2Cr2O3+3O2����
�ʴ�Ϊ��4CrO3
2Cr2O3+3O2����
��3������NaClΪ����ʣ�����������NaCl����ǿ��Һ�ĵ����������ʴ�Ϊ����ǿ��Һ�ĵ���������
����Һ��c��OH-��=
=
mol/L=
mol/L������Һ��c��Cr3+��=
=
=3.0��10��6mol/L��
�ʴ�Ϊ��3.0��10��6��
�ʴ�Ϊ��4CrO3+3CH3CH2OH+12H+�T4Cr3++3CH3COOH+9H2O��
��2������CrO3������Ϊ100g����CrO3�и�Ԫ�ص�����Ϊ��100g��
| 52 |
| 52+16��3 |
| 52 |
| 52 |
| 42.67 |
| 16 |
��B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ
| 52 |
| 52 |
| 24 |
| 16 |
| ||
�ʴ�Ϊ��4CrO3
| ||
��3������NaClΪ����ʣ�����������NaCl����ǿ��Һ�ĵ����������ʴ�Ϊ����ǿ��Һ�ĵ���������
����Һ��c��OH-��=
| 3 |
| ||
| 3 |
| ||
| 3 | 2.0��10-25 |
| KspCr(OH)3 |
| C3(OH-) |
| 6.0��10-31 |
| 2.0��10 -25 |
�ʴ�Ϊ��3.0��10��6��
���������⿼����������ԭ��Ӧ��Ksp���йؼ��㣬�Լ�ͼ���������Ŀ�Ѷ��еȣ�ע���ͼ��ķ��������ݵĴ�����

��ϰ��ϵ�д�
�����Ŀ
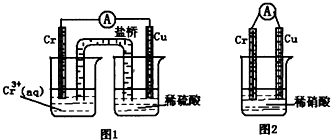
 �����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
�����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�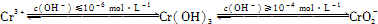
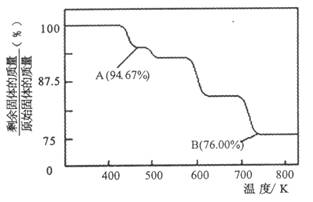

 Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O