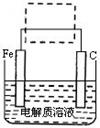
��Ŀ����
��ͭ��CuFeS2������ȡͭ���仯�������Ҫԭ��֮һ�������Ʊ������Ļ����
��1��ұ��ͭ�ķ�ӦΪ�� 8CuFeS2+21O2 8Cu+4FeO+2Fe2O3+16SO2
8Cu+4FeO+2Fe2O3+16SO2
��CuFeS2��Fe�Ļ��ϼ�Ϊ+2����Ӧ�б���ԭ��Ԫ���� ����Ԫ�ط��ţ���
��2�����û�ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
��ȥAl3+�����ӷ���ʽ�� ��
��3��Ϊ��֤¯���к���FeO���Ƚ�¯����ϡ���ܽ⣬�������������¼����ʵ��������������ṩ���Լ���ϡ���ᡢϡ���ᡢKSCN��Һ������KMnO4��Һ��NaOH��Һ����ˮ��
��ѡ�Լ�Ϊ �� ��֤��¯���к���FeO��ʵ������Ϊ ��
��4����ƽ������Ӧ�����ӷ���ʽ�������������������ѡ������[ ]��
Fe2++ H++ [ ] �� Fe3++ [ ]+ H2O
��5�����֤��¯�����Ƿ���FeO����¯������ϡ���ᣬÿ����1molFeO���ɲ������������� L����״������������Ļ�ԭ����ֻ��NO����
��6��Fe2O3������ΪȾ�ϣ�Ҳ�ɽ�һ���Ƶþ�ˮ��Fe2(SO4)3, Fe2(SO4)3�ľ�ˮԭ���� ������Ӧ�ķ���ʽ��ʾ����
��1��Cu��O
��2��Al3++4OH-=2H2O+AlO2-
��3��ϡ���ᡢKMnO4��Һ��ϡ�����ȡ¯��������ҺʹKMnO4��Һ��ɫ��
��4��5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��5��7.47 L
��6��Fe3++3H2O  Fe(OH)3�����壩+3H+
Fe(OH)3�����壩+3H+
���������������1����Ӧ8CuFeS2+21O2  8Cu+4FeO+2Fe2O3+16SO2�У����ϼ۽���Ԫ��Cu��O�ڷ�Ӧ�б���ԭ�����ϼ�����Ԫ��Fe��S���ڵ�������Fe2O3��SO2���������
8Cu+4FeO+2Fe2O3+16SO2�У����ϼ۽���Ԫ��Cu��O�ڷ�Ӧ�б���ԭ�����ϼ�����Ԫ��Fe��S���ڵ�������Fe2O3��SO2���������
��2��Al3++4OH-=2H2O+AlO2-
��3�����������Һ�������������ӣ�ʹ�ø��������Һ��ɫ���ʴ�Ϊ��ϡ���ᡢKMnO4��Һ��ϡ�����ȡ¯��������ҺʹKMnO4��Һ��ɫ��
��4��5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��5����Ӧ�Ļ�ѧ����ʽ��3 FeO + 10 HNO3 =" 3" Fe(NO3)3 + NO�� + 5 H2O����ÿ����1molFeO���ɲ�������������1/3��22.4L="7.47" L��
��6��Fe2(SO4)3��ˮ���ܽ����������������壬�������ʣ��ʻ�ѧ����ʽΪ: Fe3++3H2O  Fe(OH)3�����壩+3H+��
Fe(OH)3�����壩+3H+��
���㣺������ԭ��Ӧ�����ӷ�Ӧ�����ӵļ��飬��ѧ��Ӧ����ƽ����ѧ���㣬��������ʡ�

 ����������ϵ�д�
����������ϵ�д����᳧�����ջ�����(FeS2)��ȡ���ᣬʵ�����������᳧����(��Ҫ�ɷ���Fe2O3������FeS��SiO2�Ʊ��̷���
��һ��SO2��O2��Ӧ��ȡ�ķ�Ӧԭ��Ϊ��2SO2��O2 2SO3����һ�ܱ�������һ��ʱ���ڴﵽƽ�⡣
2SO3����һ�ܱ�������һ��ʱ���ڴﵽƽ�⡣
��1���÷�Ӧ��ƽ�ⳣ������ʽΪ______��
��2���÷�Ӧ�ﵽƽ��״̬�ı�־��______��
| A��v(SO2)=v(SO3) | B��������ƽ����Է����������� |
| C����������������� | D������ֵ������������ |
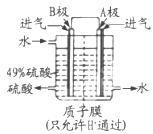
��3��B�缫�ĵ缫��ӦʽΪ______����Һ��H�����ƶ�������______����______��������ܷ�ӦʽΪ______��
�����������������̷��Ĺ�������

�ⶨ�̷���Ʒ�к�����ʵ�鲽�裺
a����ȡ5.7 g��Ʒ���ܽ⣬���250 mL����Һ��
b����ȡ25 ml������Һ����ƿ�С�
c���������ữ��0. 01 mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ���40 mL��������������ͬ���������⡣
��4���ζ�ʱ������Ӧ�����ӷ���ʽΪ����ɲ���ƽ���ӷ�Ӧ����ʽ����

��5���������ữ��KMnO4�ζ��յ�ı�־��
��6������������Ʒ�е�FeSO4.7H2O��������Ϊ______��
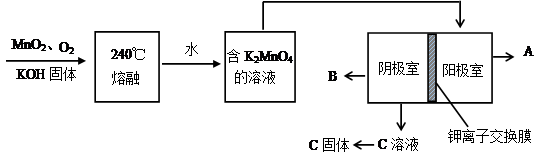
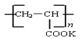 �����۱�ϩ��ص���Ľṹ��ʽΪ ��
�����۱�ϩ��ص���Ľṹ��ʽΪ ��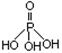 �������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȡ�
�������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȡ� Fe3O4+6SO2����3mol FeS2�μӷ�Ӧ��ת�� mol���ӡ�
Fe3O4+6SO2����3mol FeS2�μӷ�Ӧ��ת�� mol���ӡ�