��Ŀ����
��2011?ʯ��ׯ��ģ����ҵ�Ƶõĵ�������AlN����Ʒ�г���������Al4C3��Al2O3��C�����ʣ�ijͬѧ���������ʵ��ֱ�ⶨ��������AlN����Ʒ��
AlN��Al4C3����������������NH3��ǿ������Һ�е��ܽ⣩��
ʵ��ԭ����Al4C3�����ᷴӦ������CH4��AlN����ǿ�������Σ�����ǿ�����ɰ�����
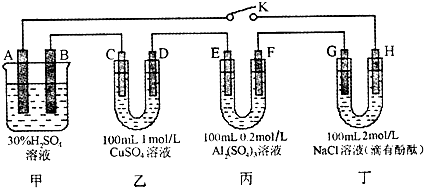
ʵ��װ�ã���ͼ��ʾ��

ʵ����̣�
����ʵ��װ�ã�����װ�õ������ԣ�
�Ƶ�Dװ�õ�����Ϊy g���ζ��ܵĶ���ΪamL��
ʢȡx g AlN��Ʒ������ƿ�У����ý������ٹرջ���
��¼�ζ��ܵĶ���ΪbmL���Ƶ�Dװ�õ�����Ϊz g��
���ݷ�����
��AlN����������Ϊ
��100%
��100%��
������ȡ�ζ�������������ʱ��Һ������ҵͣ���������������
��Al4C3����������Ϊ
��100%
��100%������ʵ�������µ�����Ħ�����ΪVm����
AlN��Al4C3����������������NH3��ǿ������Һ�е��ܽ⣩��
ʵ��ԭ����Al4C3�����ᷴӦ������CH4��AlN����ǿ�������Σ�����ǿ�����ɰ�����
ʵ��װ�ã���ͼ��ʾ��

ʵ����̣�
����ʵ��װ�ã�����װ�õ������ԣ�
�Ƶ�Dװ�õ�����Ϊy g���ζ��ܵĶ���ΪamL��
ʢȡx g AlN��Ʒ������ƿ�У����ý������ٹرջ���
K2��K3
K2��K3
������K1
K1
��ͨ����Һ©������ϡ���ᣬ����ƿ�����ʳ�ַ�Ӧ������Ӧ������ȫ�ڹرջ���K1
K1
������K3
K3
��ͨ����Һ©�����������NaOH
NaOH
���ѧʽ��������ƿ�����ʳ�ַ�Ӧ������K2��ͨ�����һ��ʱ��
��K2��ͨ�����һ��ʱ��
������ò�Ӧ���еIJ���������¼�ζ��ܵĶ���ΪbmL���Ƶ�Dװ�õ�����Ϊz g��
���ݷ�����
��AlN����������Ϊ
| 41(z-y) |
| 17x |
| 41(z-y) |
| 17x |
������ȡ�ζ�������������ʱ��Һ������ҵͣ���������������
ƫС
ƫС
���ƫ����ƫС������Ӱ�족������Al4C3����������Ϊ
| 0.048(a-b) |
| Vmx |
| 0.048(a-b) |
| Vmx |
������ʵ����̣���ʵ��װ�ú�ʵ�鲽���Ͽ�����ʵ���ԭ������������������Ʒ��Al4C3��ȫ��Ӧ����ȡ���ɵļ������壬�Ӷ��ɲ��Al4C3�İٷֺ�����������NaOH��Һ����Ʒ��AlN��ȫ��Ӧ������������ɵİ��������������������Ӷ����AlN������������
�١�ͨ����Һ©������ϡ���ᣬ��������Ʒ��Al4C3��ȫ��Ӧ����ȡ���ɵļ������壬�Ӷ����Al4C3�İٷֺ�������Ӧ�رջ���K2��K3������ K1��
�ڡ��ۡ�������NaOH��Һ����Ʒ��AlN��ȫ��Ӧ������������ɵİ���������������������Ӧ�رջ���K1������K3��
��װ���в������ְ�������K2��ͨ�����һ��ʱ�䣬�ž�װ�õİ�������װ��D��ȫ���գ�
���ݷ�����
�ݸ��ݵ�ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ����������AlN������������
��ȡ�ζ�������������ʱ��Һ������ҵͣ������ѹǿ���ڴ���ѹ���ⶨ�����������ƫС��
�߸���̼ԭ�ӵ��غ㣬Al4C3�����ʵ������ڼ�������ʵ���������֮һ�������Al4C3������������
�١�ͨ����Һ©������ϡ���ᣬ��������Ʒ��Al4C3��ȫ��Ӧ����ȡ���ɵļ������壬�Ӷ����Al4C3�İٷֺ�������Ӧ�رջ���K2��K3������ K1��
�ڡ��ۡ�������NaOH��Һ����Ʒ��AlN��ȫ��Ӧ������������ɵİ���������������������Ӧ�رջ���K1������K3��
��װ���в������ְ�������K2��ͨ�����һ��ʱ�䣬�ž�װ�õİ�������װ��D��ȫ���գ�
���ݷ�����
�ݸ��ݵ�ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ����������AlN������������
��ȡ�ζ�������������ʱ��Һ������ҵͣ������ѹǿ���ڴ���ѹ���ⶨ�����������ƫС��
�߸���̼ԭ�ӵ��غ㣬Al4C3�����ʵ������ڼ�������ʵ���������֮һ�������Al4C3������������
����⣺ʵ����̣���ʵ��װ�ú�ʵ�鲽���Ͽ�����ʵ���ԭ������������������Ʒ��Al4C3��ȫ��Ӧ����ȡ���ɵļ������壬�Ӷ��ɲ��Al4C3�İٷֺ�����������NaOH��Һ����Ʒ��AlN��ȫ��Ӧ������������ɵİ��������������������Ӷ����AlN������������
�١�ͨ����Һ©������ϡ���ᣬ��������Ʒ��Al4C3��ȫ��Ӧ����ȡ���ɵļ������壬�Ӷ����Al4C3�İٷֺ�������Ӧ�رջ���K2��K3������ K1��
�ʴ�Ϊ��K2��K3�� K1��
�ڡ�������NaOH��Һ����Ʒ��AlN��ȫ��Ӧ������������ɵİ��������������������Ӷ����AlN��������������Ӧ�رջ���K1������K3
�ʴ�Ϊ��K1��K3��
�ۡ��ɢ��з�����֪��ͨ����Һ©�������������������Һ��
�ʴ�Ϊ��NaOH��
��װ���в������ְ�������K2��ͨ�����һ��ʱ�䣬�ž�װ�õİ�������װ��D��ȫ���գ���ֹ�ⶨ�İ���������ƫС��
�ʴ�Ϊ����K2��ͨ�����һ��ʱ�䣮
���ݷ�����
�ݰ���������Ϊ��z-y��g�����ʵ���Ϊ
=
mol�����ݵ�ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ���������AlN������Ϊ
mol��41g/mol=
g����AlN����������Ϊ
��100%=
��100%��
�ʴ�Ϊ��
��100%��
��ȡ�ζ�������������ʱ��Һ������ҵͣ������ѹǿ���ڴ���ѹ���ⶨ�����������ƫС��
�ʴ�Ϊ��ƫС��
��������Ϊ��b-a��mL�����ʵ���Ϊ
=
��10-3mol������̼ԭ�ӵ��غ㣬Al4C3�����ʵ������ڼ�������ʵ���������֮һ������Al4C3������Ϊ
��
��10-3mol��144g/mol=
��10-3g��Al4C3����������Ϊ
��100%=
��100%��
�ʴ�Ϊ��
��100%��
�١�ͨ����Һ©������ϡ���ᣬ��������Ʒ��Al4C3��ȫ��Ӧ����ȡ���ɵļ������壬�Ӷ����Al4C3�İٷֺ�������Ӧ�رջ���K2��K3������ K1��
�ʴ�Ϊ��K2��K3�� K1��
�ڡ�������NaOH��Һ����Ʒ��AlN��ȫ��Ӧ������������ɵİ��������������������Ӷ����AlN��������������Ӧ�رջ���K1������K3
�ʴ�Ϊ��K1��K3��
�ۡ��ɢ��з�����֪��ͨ����Һ©�������������������Һ��
�ʴ�Ϊ��NaOH��
��װ���в������ְ�������K2��ͨ�����һ��ʱ�䣬�ž�װ�õİ�������װ��D��ȫ���գ���ֹ�ⶨ�İ���������ƫС��
�ʴ�Ϊ����K2��ͨ�����һ��ʱ�䣮
���ݷ�����
�ݰ���������Ϊ��z-y��g�����ʵ���Ϊ
| (z-y)g |
| 17g/mol |
| z-y |
| 17 |
| z-y |
| 17 |
| 41(z-y) |
| 17 |
| ||
| xg |
| 41(z-y) |
| 17x |
�ʴ�Ϊ��
| 41(z-y) |
| 17x |
��ȡ�ζ�������������ʱ��Һ������ҵͣ������ѹǿ���ڴ���ѹ���ⶨ�����������ƫС��
�ʴ�Ϊ��ƫС��
��������Ϊ��b-a��mL�����ʵ���Ϊ
| (b-a)��10-3L |
| VmL/mol |
| b-a |
| Vm |
| 1 |
| 3 |
| b-a |
| Vm |
| 48(b-a) |
| Vm |
| ||
| xg |
| 0.048(a-b) |
| Vmx |
�ʴ�Ϊ��
| 0.048(a-b) |
| Vmx |
�����������ʵ��ԭ���������������⡢��ѧ���㡢������ɵIJⶨ�ȣ��ѶȽϴ��Ƕ�����֪ʶ���ۺ����ã���Ҫѧ��������ʵ�Ļ���֪ʶ��������⡢������������������ʵ��ԭ���ǽ��Ĺؼ���

��ϰ��ϵ�д�
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
�����Ŀ