��Ŀ����
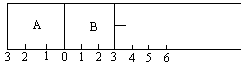
��6�֣���ͼ��ʾ��ij�����ָ���A��B�����֣�A�����̶����䣬B�п��ƶ��Ļ���������A�г���2molSO2��1molO2����B�г���2 molSO3��1molN2������ͬ�����·������淴Ӧ��2SO2(g)��O2(g) ![]() 2SO3(g)����������Ҫ����գ�
2SO3(g)����������Ҫ����գ�
 |
���¶Ȳ����£��̶�����λ����3���������ﵽƽ�����A����ѹǿΪPA��B����ѹǿΪPB����PA��PB��ѹǿ��PA PB (�������������������������ͬ)��A��SO2��Ũ�� B��SO2��Ũ�ȡ�
�����������Ƶ�5�����ﵽƽ���B��SO3Ϊx mol��A��SO3Ϊy mol����x y (���������������������)��
(1) <��2�֣� = ��2�֣���(2)< ��2�֣�

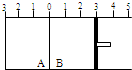 ��ͼ��ʾ���¶Ȳ���ʱij�����ָ���A��B�����֣�A��B����֮����ܷ��������������ƶ���B�����Ҳ��п��ƶ��Ļ���������A�����г���2mol SO2��1mol O2����B�����г���2mol SO3��g����1mol N2������ͬ�¶Ⱥ�ѹǿ�¿ɷ������淴Ӧ��2SO2��g��+O2��g��?2SO3��g��������˵����ȷ���ǣ�������
��ͼ��ʾ���¶Ȳ���ʱij�����ָ���A��B�����֣�A��B����֮����ܷ��������������ƶ���B�����Ҳ��п��ƶ��Ļ���������A�����г���2mol SO2��1mol O2����B�����г���2mol SO3��g����1mol N2������ͬ�¶Ⱥ�ѹǿ�¿ɷ������淴Ӧ��2SO2��g��+O2��g��?2SO3��g��������˵����ȷ���ǣ�������| A��ƽ���A��B�������������� | B��ƽ���A��B�������е�SO3������������ | C��ƽ���A��B�������е�SO2��ת������� | D��ƽ���������SO2�����ʵ�����A��B |
| |||||||||||||||