��Ŀ����
����������һ����;ʮ�ֹ㷺�Ļ���ԭ�ϡ���ҵ����Ҫͨ������Ȼ��Ʊ�����Һ�ķ�������������ƣ��ҹ����ȼҵ������������ӽ���Ĥ���ۡ�
(1)���ӽ���Ĥ����һ����ý���������������ԭ����__________________________________��
����һ����̼�����Ƴɡ������ӽ���Ĥ�ѵ��۸��������Һ������ң���������____________________________________��
(2)Ϊʹ����Ȼ��Ƶ����ʼӿ죬���д�ʩ���е���__________��
a����������̼���������
b����߱����Ȼ�����Һ���¶�
c���Ӵ�������������ľ���
d����ߵ��ʱ��Դ��ѹ
(3)�����ij���ӽ���Ĥ���۵Ģٵ��ʱ��ѹ����ԭ����2�����ڵ��ʱ�ĵ���ǿ������ԭ����2�����۵��ʱ�¶ȴ�30 ����ߵ�60 �棬��������һ����ﵽԭ����2������________________��
(1)����������Cl2����ʴ�ѡ����ܷ�ֹH2��Cl2��ϱ�ը�����ܱ���Cl2��NaOH��Ӧ
(2)abd��(3)��
����

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д� �߽�������ϵ�д�
�߽�������ϵ�д��ؿ�����Ԫ�ر�ͭԪ�غ����ߺܶ࣬������ұ����ͭ������ܶ��ꡣ�����Ľ�����
| A��ͭ�����ԭ��������ѻ�ԭ |
| B��ͭ����ɫ������֣�������ɫ��dz�������� |
| C����ʯ�ڵ�����أ�ͭ����dz�������������������ѿ��� |
| D��ͭ�������ԭ�������� |
��Դ��������������ᷢչ������أ����й�����Դ���������õ�˵���У�����Ϊ˵������ȷ���ǣ� ��
| A���������̫���� |
| B��������˿������÷��ܡ�ˮ�ܡ������ܡ���ϫ�� |
| C����������ȫ�����������ܡ����� |
| D����Դ����ͨ����ѧ��Ӧ��õ� |
(1)���з�Ӧԭ�������Ϲ�ҵұ������ʵ���������(����)��
A��2HgO 2Hg+O2�� 2Hg+O2�� | B��Fe3O4+4CO 3Fe+4CO2 3Fe+4CO2 |
C��2MgO 2Mg+O2�� 2Mg+O2�� | D��2Ag2O 4Ag+O2�� 4Ag+O2�� |


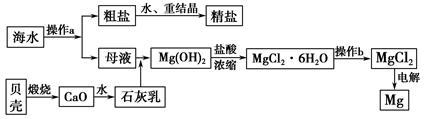
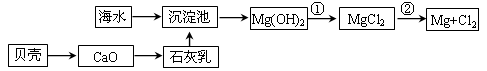
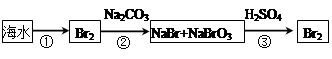


 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��