��Ŀ����
(12��)��ѧ��һֱ�����ڡ��˹��̵����ķ����о���
(1)�ϳɰ���ԭ��Ϊ��N2(g)+3H2(g) 2NH3(g)
2NH3(g)  H=-92��4 kJ��mol���÷�Ӧ�������仯��ͼ��ʾ��
H=-92��4 kJ��mol���÷�Ӧ�������仯��ͼ��ʾ��
���ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯�� (���������С�����䡱)��
�ڽ�0��3 mol N2��0��5 mol H2�������������ܱ������У���һ�������´ﵽƽ�⣬�������������ѹǿ��Ϊԭ���� ����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
| A���������а�ԭ�����ٳ���ԭ���� | B�����������ٳ���һ����H2 |
| C���ı䷴Ӧ�Ĵ��� | D��Һ�������������� |
2N2(g)+6H2O(1)
 4NH3(g)+3O2(g)
4NH3(g)+3O2(g)  H="+1530" kJ��mol
H="+1530" kJ��mol��֪��H2O(1)=H2O(g)
 H=+44��0 kJ��mol
H=+44��0 kJ��mol��2N2(g)+6H20(g)
 4NH3(g)+302(g)
4NH3(g)+302(g)  H = kJ��mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK= �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)��
H = kJ��mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK= �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)��
��12�֣���1���� ��С �� 30% ��A D
��2�� +1266 �� 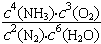 �� ���� ��ÿ��2�֣���12�֣�
�� ���� ��ÿ��2�֣���12�֣�
����

��ϰ��ϵ�д�
�����Ŀ


