��Ŀ����
ij��ɫ��Һ�п��ܺ���Na����SO42�D��SO32�D��Cl�D��Br�D��CO32�D�е������֣����ν�������ʵ�顣�۲쵽�������£�����pH��ֽ���飬��ҺpH>7��������Һ�еμ���ˮ,������������ټ���CCl4�����á�CCl4��ʳ�ɫ���÷�Һ©����Һ�������Һ���ˮ��Һ�м���Ba(NO3)2��HNO3�Ļ��Һ��ֻ�а�ɫ���������ˣ�������Һ�м���AgNO3��HNO3�Ļ��Һ���а�ɫ����������
�ش��������⣺
��1��ԭ��Һ�п϶����е������� ���϶�û�е������� ��
��2������۸���Ba(NO3)2������Ļ��Һ�����жϢ�����Ӱ�죿
��
��3��д��������е����ӷ���ʽ ��
��1��Br����SO32����Na��CO32��
��2����Ӱ��
��3��Cl2+2Br����Br2+2Cl����SO32�D+Cl2+H2O��2H��+2Cl��+SO42��
����:
���ݢ�֪������CO32�D��SO32�D�����ݢڿ�ȷ����CO32�D����϶���SO32�D��Br�D�����õ���غ㣬��ȷ����Na�����ۺܱ͢�����Һ��������Cl�D��SO42�D��������������ˮ����Cl�D����ȷ����SO42�D��������SO32�Dת���ģ���SO42�DҲ����ȷ���Ƿ�ԭ��Һ��������

��2011?������һģ��ij��ɫ��Һ�п��ܺ��� ��Na+����Ba2+����Cl-����Br-����SO42-����SO32-�����е������֣���˳���������ʵ�飬��ÿ�������Լ����������۲쵽���������£�
|
�ٿ϶�����I-���ڿ϶�����Cu2+���ۿ϶�����SO32-�ܿ��ܺ���I-��
| A���٢� | B���٢ڢ� | C���ۢ� | D���ڢۢ� |
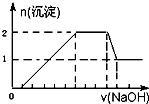 ij��ɫ��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�������еļ��֣��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ������˵��һ����ȷ���ǣ�������
ij��ɫ��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�������еļ��֣��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ������˵��һ����ȷ���ǣ�������| A��һ������H+��Mg2+��Al3+��NH4+��һ��������Na+��SO42-��Fe3+ | B��һ������H+��Al3+��NH4+��SO42-�����ܴ���Na+��Mg2+ | C����Һ��c��H+����c��Al3+����c��Mg2+��Ϊ1��1��1 | D����Һ��c��H+����c��SO42-��Ϊ2��9 |