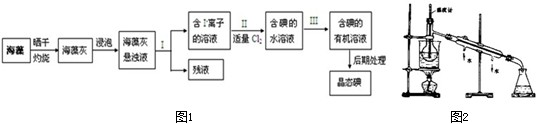
��Ŀ����
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ������պ�ҽ��еĵ��Ե�������ʽ���ڣ�ijʵ��С��ͬѧΪ����֤�����к��е⣮������ȡ�Ⲣ���㺣���еĺ����������������ʵ�飺
��1���ڽ��в����֮ǰ�����dz�ȡ��a g�ɺ�������ͬѧ����������ȼ��飮Ȼ����ˮϴ�����������У���������������ȫ�ɻҽ�������ͬѧ��Ϊ�IJ������鲻�������������� ��
��2������ȴ�ĺ�����ת����С�ձ���������������ݺ����ҵ��Լ��� ��
��3��������������漰��һϵ�в��������� ��
��4��������м�ͬѧ����ҺA���ȵμӼ��ε�����Һ��δ�������Ա仯���ٵμ�������ϡ���ᷢ����Һ��ɫ��������д���˷�Ӧ�����ӷ���ʽ�� ����ͬѧ�۲����ͬѧ��ʵ���Ϊ��ʹI-��Ӧ�ø���ȫ�����������ѡ�õ��������� ��
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2��Һ
��5���ڲ��ɢ���ȡ��Ĺ����У���ͬѧ����ҺB��������x�У��������м���һ�������Լ�Y�������ã�д������X������ ��
��6���������ҺC����ȡ�Ⲣ�����Լ�Y�IJ��������� ������ȡ�õ�bg�⣬��˺����к���Ԫ�ص����������� ��
���𰸡���������1��������ˮ����һ��ʱ�䣬�⻯�����ܽ���ˮ�У�
��2���������ҷ�������ˮ�н��ݣ����Խ�����ˮ�IJ��ֺ��������ʷ��룻
��3����������Һ��IJ����ǹ��ˣ�
��4�����������Ե����ʿ��Խ�����������Ϊ���ʵ⣬˫������ɫ��������
��5��ʵ����ȡ��������������ѡ���Һ©����
��6������л���ķ�����Բ�����������Ԫ���غ��������Ԫ�ص�����������
����⣺��1���������ȼ��飮Ȼ����ˮϴ������������ʱ��������ˮ����һ��ʱ�䣬�����⻯�����ܽ���ˮ�У�ʹ�õ�Ԫ�صĺ������٣�
�ʴ�Ϊ����ˮϴ��ʱ���⻯�����ܽ���ˮ�У���ʹ�ú����е�һЩ��Ԫ�ؼ��٣�
��2���������ҷ�������ˮ�н��ݣ��ʴ�Ϊ������ˮ��
��3�����������ԵĹ���Ϳ����Եĺ��е�Ԫ�ص�Һ��IJ����ǹ��ˣ��ʴ�Ϊ�����ˣ�
��4�����������Ե����ʿ��Խ�����������Ϊ���ʵ⣬˫������ɫ�������������Ի����£����������ӵ�ʵ���ǣ�H2O2+2H++2I-=2H2O+I2����ԭ������ˮ������������ʣ�
�ʴ�Ϊ��H2O2+2H++2I-=2H2O+I2��D��
��5��ʵ����ȡ��������������ѡ���Һ©�����ʴ�Ϊ����Һ©����
��6������л���ķ�����Բ�����������ȡ�õ�bg�⣬���Ԫ�ص�Ԫ�ص���������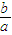 ×100%���ʴ�Ϊ��
×100%���ʴ�Ϊ��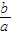 ��
��
���������⿼�������ʵķ�����ᴿ���ѶȲ���ѧϰ��ע��������ȡ�IJ�����
��2���������ҷ�������ˮ�н��ݣ����Խ�����ˮ�IJ��ֺ��������ʷ��룻
��3����������Һ��IJ����ǹ��ˣ�
��4�����������Ե����ʿ��Խ�����������Ϊ���ʵ⣬˫������ɫ��������
��5��ʵ����ȡ��������������ѡ���Һ©����
��6������л���ķ�����Բ�����������Ԫ���غ��������Ԫ�ص�����������
����⣺��1���������ȼ��飮Ȼ����ˮϴ������������ʱ��������ˮ����һ��ʱ�䣬�����⻯�����ܽ���ˮ�У�ʹ�õ�Ԫ�صĺ������٣�
�ʴ�Ϊ����ˮϴ��ʱ���⻯�����ܽ���ˮ�У���ʹ�ú����е�һЩ��Ԫ�ؼ��٣�
��2���������ҷ�������ˮ�н��ݣ��ʴ�Ϊ������ˮ��
��3�����������ԵĹ���Ϳ����Եĺ��е�Ԫ�ص�Һ��IJ����ǹ��ˣ��ʴ�Ϊ�����ˣ�
��4�����������Ե����ʿ��Խ�����������Ϊ���ʵ⣬˫������ɫ�������������Ի����£����������ӵ�ʵ���ǣ�H2O2+2H++2I-=2H2O+I2����ԭ������ˮ������������ʣ�
�ʴ�Ϊ��H2O2+2H++2I-=2H2O+I2��D��
��5��ʵ����ȡ��������������ѡ���Һ©�����ʴ�Ϊ����Һ©����
��6������л���ķ�����Բ�����������ȡ�õ�bg�⣬���Ԫ�ص�Ԫ�ص���������
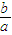 ×100%���ʴ�Ϊ��
×100%���ʴ�Ϊ��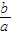 ��
�����������⿼�������ʵķ�����ᴿ���ѶȲ���ѧϰ��ע��������ȡ�IJ�����

��ϰ��ϵ�д�
 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�
�����Ŀ
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڣ�ʵ���ҴӺ�������ȡ����������£�
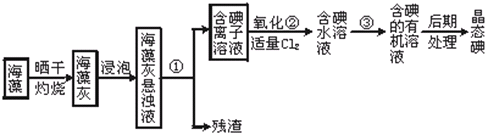
��1��ָ����ȡ��Ĺ������й�ʵ��������� �� ��
��2��д�����̢����йط�Ӧ�����ӷ���ʽ
��3����ȡ��Ĺ����У��ɹ�ѡ����л��Լ���
A������ B���� C�����Ȼ�̼ D���ƾ�

��1��ָ����ȡ��Ĺ������й�ʵ��������� ��
��2��д�����̢����йط�Ӧ�����ӷ���ʽ
��3����ȡ��Ĺ����У��ɹ�ѡ����л��Լ���
A������ B���� C�����Ȼ�̼ D���ƾ�
| �л��Լ� | �ƾ� | ���Ȼ�̼ | �� | ���� |
| ���� | �� | �� | �� | �� |
| ˮ���� | ��ˮ������Ȼ��� | ��ˮ�������� | ��ˮ�������� | ��ˮ������Ȼ��� |