��Ŀ����
��15�֣�ijǿ������ҺX����Ba2+��Al3+��NH4+��Fe2����Fe3+��CO32-��SO32-��SO42-��NO3-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飬ʵ���������£�
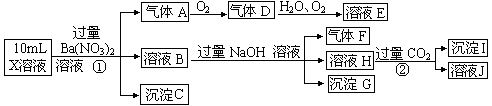
����������Ϣ���ش��������⣺
��1�����������У���ҺX�г�H����϶����е�������____________________������ȷ���Ƿ��е�������___________����Ҫ��֤�������Ƿ���ڣ���ɿ��Ļ�ѧ������_______________________
��2������G�Ļ�ѧʽΪ_____________��
��3��д���й����ӷ���ʽ��
��������A________________________ ��
��_________ ��

����������Ϣ���ش��������⣺
��1�����������У���ҺX�г�H����϶����е�������____________________������ȷ���Ƿ��е�������___________����Ҫ��֤�������Ƿ���ڣ���ɿ��Ļ�ѧ������_______________________
��2������G�Ļ�ѧʽΪ_____________��
��3��д���й����ӷ���ʽ��
��������A________________________ ��
��_________ ��
��1��Al3+ NH4+ Fe2+ SO42- Fe3+
ȡ������Һ���Թ��У��μ���KSCN��Һ������Һ��ΪѪ��ɫ����֤����Һ�к���Fe3+����֮���ޡ�
��2��Fe(OH)3
��3����3Fe2++NO3-+4H+==3Fe3++NO��+2H2O
��[Al(OH)4]-+CO2==Al(OH)3��+HCO3-
ȡ������Һ���Թ��У��μ���KSCN��Һ������Һ��ΪѪ��ɫ����֤����Һ�к���Fe3+����֮���ޡ�
��2��Fe(OH)3
��3����3Fe2++NO3-+4H+==3Fe3++NO��+2H2O
��[Al(OH)4]-+CO2==Al(OH)3��+HCO3-
��1����ǿ������Һ������CO32-��SO32-�����Կ϶�����Fe2���������ᷴӦ�Ų�������A ������A ��NO����ҺB ����NH4+��Fe3+��Al3+������C ��BaSO4�����ԣ�ǿ������ҺX�г�H����϶����е������ǣ�Al3+ NH4+ Fe2+ SO42-������ȷ���Ƿ��е�������Fe3+����Ҫ��֤Fe3+�Ƿ���ڣ��䷽����ȡ������Һ���Թ��У��μ���KSCN��Һ������Һ��ΪѪ��ɫ����֤����Һ�к���Fe3+����֮���ޡ�
��2������G�Ļ�ѧʽΪFe(OH)3
��3����������A�����ӷ���ʽ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O
��AlO2����2H2O+CO2=Al(OH)3��+HCO3-
��2������G�Ļ�ѧʽΪFe(OH)3
��3����������A�����ӷ���ʽ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O
��AlO2����2H2O+CO2=Al(OH)3��+HCO3-

��ϰ��ϵ�д�
�����Ŀ
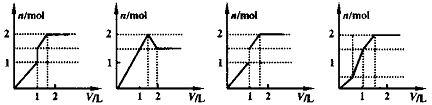
 ��3����һƿ�������Һ�����п��ܺ���H+��NH+4��Mg2+��Ba2+��Al3+��I-��NO-3��CO2-3��SO2-4��AlO-2��ȡ����Һ��������ʵ�飺
��3����һƿ�������Һ�����п��ܺ���H+��NH+4��Mg2+��Ba2+��Al3+��I-��NO-3��CO2-3��SO2-4��AlO-2��ȡ����Һ��������ʵ�飺 ��Һ���а�ɫ�������ɣ�֤��______���ڣ����ų�________���ڡ�
��Һ���а�ɫ�������ɣ�֤��______���ڣ����ų�________���ڡ�