��Ŀ����
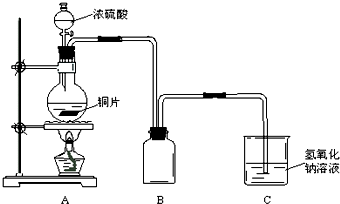
ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飺

��ش��������⣺
��1��B�������ռ�ʵ���в��������װ�ã���δ�����ܻ�ȫ���뽫װ��ͼ����������
��2��ʵ��������ȡ6.4gͭƬ��12mL18mol��L-1Ũ�������Բ����ƿ�й��ȣ�ֱ����Ӧֹͣ���������ƿ�л���ͭƬʣ�࣬��С���е�ͬѧ��Ϊ����һ����������ʣ�ࡣ
��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
______________________________________________________________________��
ʵ��������m gͭ�μ��˷�Ӧ������___________mol���ᱻ����������ת����ĿΪ_______mol��
�������Լ��У���֤����Ӧֹͣ����ƿ��������ʣ�����__________����д��ĸ��ţ���
A.��������Һ B.�Ȼ�����Һ C.���� D.̼������Һ
��Ϊʲô��һ����������ʣ�൫δ��ʹͭƬ��ȫ�ܽ⣿����Ϊ��ԭ����
_________________________________________________________________ _����3��Ϊ�˲ⶨʣ����������ʵ���Ũ�ȣ�����ȤС�����������ʵ�鷽����
����һ����װ��A���������建��ͨ���ѳ�������װ�м�ʯ�ҵĸ���ܣ���Ӧֹͣ���ٴγ�������������������յĶ�������
����������װ��A���������建��ͨ���������������ữ�ĸ��������Һ���ټ����������Ȼ�����Һ�����ˡ�ϴ�ӡ�����Ƶó������������Ƕ�������ת��Ϊ���ᱵ������������
����������ͭ��Ũ����ķ�Ӧ��������װ��A�м���������п�ۣ�����ˮ����ò������������ΪV L���ѻ���Ϊ��״������
ʵ���ϣ����Ϸ���һ����������ȡ������˵��ԭ��
����һ ��
������ ��
��������д��ʣ����������ʵ���Ũ�ȵļ���ʽ�����跴Ӧ����Һ�������Ϊ12mL�� _________________________ _��
��1�������������������ƿ�ף�ͼ�ԣ�
��2���� Cu + 2H2SO4(Ũ) ![]() CuSO4 + SO2��+ 2H2O�� m/64 �� m/32
CuSO4 + SO2��+ 2H2O�� m/64 �� m/32
��D ��ϡ�����ͭ��Ӧ
��3������һ�������������к���ˮ������������е������������SO2������������ƿ�еĶ�����������ȫ�ų���������������������һ�����Ǹ��������Һ�����ữ���õ��������Ȼ�����Ӧ�������� �������� V/(22.4��0.012) mol/L

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
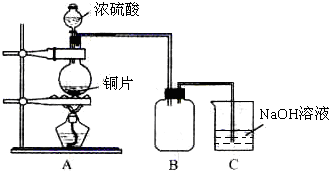
 ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ�������ͼ��ʾװ�ý����й�ʵ�飮��ش�
ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ�������ͼ��ʾװ�ý����й�ʵ�飮��ش� ij��ѧ������ȤС��Ϊ̽�������巢����Ӧ�ķ�Ӧ���Ͳ���ȡ�������屽����������ʵ�飮�����Ҫ��ش�������⣮
ij��ѧ������ȤС��Ϊ̽�������巢����Ӧ�ķ�Ӧ���Ͳ���ȡ�������屽����������ʵ�飮�����Ҫ��ش�������⣮