��Ŀ����
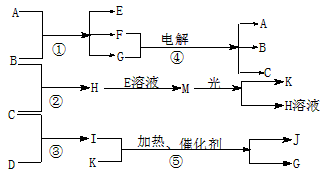
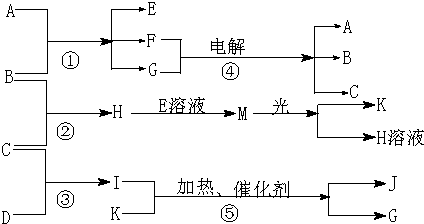
����A�ǻ����B��C��D��K���ǵ��ʣ���Ӧ�ڡ��ݶ��dz����Ĺ�ҵ�����ķ�Ӧ��
����д���пհף�
(1)д���������ʵĻ�ѧʽ��B__________��J_________��
(2)д�����з�Ӧ�����ӷ���ʽ��
��H+E(��Һ)��M__________________����I����G���ּ���__________________��?
(3)��ͨ��״���£���1 g C������B������ȼ������H����ʱ�ų�92.3 kJ��������2 mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ__________________?
(1)д���������ʵĻ�ѧʽ��B__________��J_________��
(2)д�����з�Ӧ�����ӷ���ʽ��
��H+E(��Һ)��M__________________����I����G���ּ���__________________��?
(3)��ͨ��״���£���1 g C������B������ȼ������H����ʱ�ų�92.3 kJ��������2 mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ__________________?
(1)B��Cl2��J��NO
(2)�� H+ + ClO-= HClO���� NH3+ H2O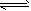 NH3��H2O
NH3��H2O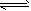 NH4++OH-
NH4++OH-
(3)2HCl(g)=H2(g)+Cl2(g)����H= +184.6 kJ��mol-1?
(2)�� H+ + ClO-= HClO���� NH3+ H2O
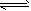 NH3��H2O
NH3��H2O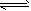 NH4++OH-
NH4++OH- (3)2HCl(g)=H2(g)+Cl2(g)����H= +184.6 kJ��mol-1?

��ϰ��ϵ�д�
�����Ŀ
 ?
? NH3?H2O
NH3?H2O NH4++OH-
NH4++OH-