��Ŀ����
��������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��
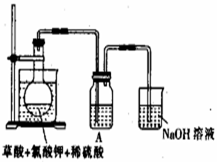
��1��ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ
![]()
![]()
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� (����ĸ)��
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ
��2����ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������(![]() )������ˮ��ClO2��
)������ˮ��ClO2��![]() �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:
![]()
![]() ������4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ![]() ����Һ�е�
����Һ�е�![]() ��ԭΪ
��ԭΪ![]() �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
��������ˮ��![]() �ĺ������꣬�������м���������
�ĺ������꣬�������м���������![]() ��
��![]() ��ԭΪ
��ԭΪ![]() ����÷�Ӧ����������Ϊ (�ѧʽ)
����÷�Ӧ����������Ϊ (�ѧʽ)
��1����ʹ��Ӧ�������У�����ֹ�¶ȹ�������ը �¶ȼ�
��b ��6.75g
��2����25mL��ʽ�ζ���
�ڵ�����Һ����ɫ��ȥ
��![]()
��Fe��OH��3


 NCl3+3H2��������NCl3�е�Ԫ��Ϊ+3�ۡ�
NCl3+3H2��������NCl3�е�Ԫ��Ϊ+3�ۡ�