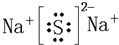��Ŀ����
�������ж�����Ԫ�����ʵ��й����ݣ�
| Ԫ�ر�� | a | b | c | d | e | f | g | h |
| ԭ�Ӱ뾶��nm | 0.037 | 0.099 | 0.077 | 0.110 | 0.104 | 0.143 | 0.066 | 0.186 |
| ����ϼۻ���ͻ��ϼ� | +1 | -1 | +4 | +5 | +6 | +3 | -2 | +1 |
�����ϱ��ش��������⣺
��1������c�����ܾ��е������� ��
A���������õĵ����� B���۷е�ܸ� C��Ӳ�Ƚ�С D�����뵥��a��Ӧ
��2��д��e��h��Ӧ������ĵ���ʽ ��
��3��g��h��Ӧ����һ����ǿ�����Ե����ʣ�д����������ˮ��Ӧ�Ļ�ѧ����ʽ
![]() ���÷�Ӧ�Ļ�ԭ����(�û�ѧʽ��ʾ) ��
���÷�Ӧ�Ļ�ԭ����(�û�ѧʽ��ʾ) ��
��4��a��c�γɵ���Է���������С�����ʣ�4g��������ȫȼ�������ȶ�������ʱ�ų�222��55kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ
��1��D
��2��![]()
��3��2Na2O2+2H2O=4NaOH+O2�� Na2O2
��4��CH4��g��+2O2��g��=CO2��g��+2H2O��l�� ��H=-890.2kJ��mol-1

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ