��Ŀ����
���ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O,Ϊ�ⶨxֵ����������ʵ�飺�ٳ�ȡW g�����ᾧ�壬�������Ƴ�100.0 mLˮ��ҺΪ����Һ��
��ȡ25.0 mL����Һ������ƿ�У��ټ���������ϡH2SO4��
����Ũ��Ϊa mol��L��1��KMnO4����Һ���еζ����ζ�ʱ�����ķ�ӦΪ��
2KMnO4+5H2C2O4+3H2SO4===K2SO4+2MnSO4+10CO2��+8H2O
��ش�
(1)�ζ�ʱ����KMnO4��Һװ��_________�ζ����У�����ʱ��_________������ƿ��
(2)���ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��_________(�ƫ��ƫС�����䡱)��
(3)����ζ��յ�ʱ����ȥV mL KMnO4��Һ������������Һ�����ʵ���Ũ��Ϊ_________mol��L��1��
������(1)����KMnO4�������ԣ����������ܣ�����ֻ��װ����ʽ�ζ��ܡ�
(2)��Ϊx=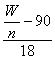 ,����nΪ��������ʵ���������KMnO4Ũ�ȼ�С����ʹ���ĵ�KMnO4��������ʵ���nֵ��ƫ����x��ƫС��
,����nΪ��������ʵ���������KMnO4Ũ�ȼ�С����ʹ���ĵ�KMnO4��������ʵ���nֵ��ƫ����x��ƫС��
(3)2KMnO4 �� 5H2C2O4
2 5
a��V c��25
����c=![]() =
=![]() mol��L��1
mol��L��1
�𰸣�(1)��ʽ �� (2)ƫС
(3)![]()

��ϰ��ϵ�д�
�����Ŀ
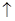 +8H2O
+8H2O