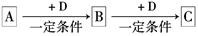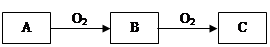��Ŀ����
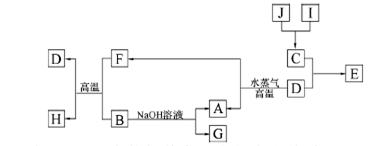
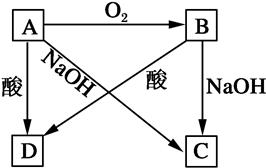
��ѧ��ѧ��������A��һ�������·������·�Ӧ��A+B��E+F+H2O��δ��ƽ��
��1����AΪС�մ�FΪ���塣�÷�Ӧ�����ӷ���ʽΪ ��
��2����AΪ�Ϻ�ɫ�������ʣ�����F��������λ��ͬһ����Ķ�����Ԫ����ɡ���Ӧ�Ļ�ѧ����ʽΪ_____________________��
��3����A�Ǵ��������Ҫ�ɷ֣�B�����ᡣд����Ӧ�Ļ�ѧ����ʽΪ ��
��4����AΪ����ɫ���嵥�ʣ�F�ļ�����Һ���շ�����SO2�����ӷ���ʽΪ ��
��1��HCO3����H+��H2O��CO2����2�֣�
��2��Cu+2H2SO4=CuSO4+SO2+2H2O��2�֣�
��3��Fe3O4��8HCl��2FeCl3��FeCl2��4H2O(��Fe3O4��8H+��2Fe3+��Fe2+��4H2O)��2�֣�
��4��2OH��+ClO��+SO2��Cl��+SO42��+H2O��2�֣�
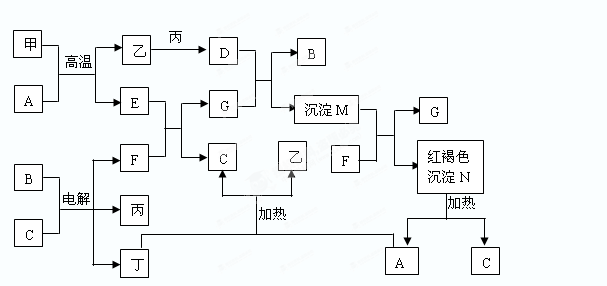
���������������1����AΪС�մ�������Ƴ�A����ķ�Ӧ����2����AΪCu������FΪSO 2��������Ƴ�Cu��Ũ����ķ�Ӧ����3����AΪ������ӦΪFe3O4��8HCl��2FeCl3��FeCl2��4H2O��4����AΪ����ɫ����Cl2�������д��Cl2��SO2�ķ�Ӧ�����õ��Ӻ͵���غ㡣
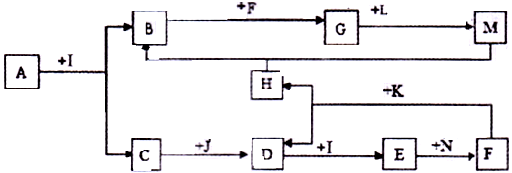
���㣺�����ƶϼ���ط���ʽ����д

��ϰ��ϵ�д�
�����Ŀ