��Ŀ����
��08����һ�ж�ģ�������16�֣�B��һ�����岻��ȱ�ٵ���ɫ��ζ��Һ�壻C��һ���д��ԵĻ����E��һ����ɫ����ζ���ж����壬�������й�ϵͼ����ɱ��⣺

(1��д��B��C�Ļ�ѧʽ��B ��C
��2��E��N2�ṹ���ƣ���д��E�ĵ���ʽ��E

A�������¶� B������ѹǿ C���������
��4����G��һ�ֵ���ɫ���嵥�ʣ�16�˵�G��������A��Ӧ�ų�������ΪQ kJ��Q>0����д�������Ӧ���Ȼ�ѧ����ʽ______
��G��һ����̬���ʣ�H��ˮ��Һ���Ժ��л���I��Ӧʹ��Һ����ɫ����д��G��I�ķ���ʽ�� �� ��H��ˮ��Һ�����ԣ����û�ѧ����ʽ��ʾ�����Ե�ԭ��
�𰸣���ÿ��2�֣�16�֣���1��H2O Fe3O4
![]() ��2�� C O
��2�� C O
��3��BC����ѡ��1�֣���ѡ�����֣�
��4��S��s����Fe��s����FeS��s���� ��H����2Q kJ?mol��1������ʽҪ��ȫ�ԲŸ��֣�
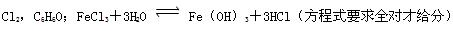

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ
 (1)��ҵ��ȡD���ʵĻ�ѧ����ʽΪ
(1)��ҵ��ȡD���ʵĻ�ѧ����ʽΪ  ��1����ͬѧʵ�����Ҫ�����У��ٹ��� �ڼ���������CaCl2��Һ �۽�����С�ĺ�ɡ���������ù�������Ϊng �ܳ�ȡmg��Ʒ������������ˮ ��ϴ�ӳ���2�D3�Ρ���ͬѧ��ȷ�IJ�������Ϊ ������ţ����������̼���Ƶ���������Ϊ ��ֻ���г�����ʽ����
��1����ͬѧʵ�����Ҫ�����У��ٹ��� �ڼ���������CaCl2��Һ �۽�����С�ĺ�ɡ���������ù�������Ϊng �ܳ�ȡmg��Ʒ������������ˮ ��ϴ�ӳ���2�D3�Ρ���ͬѧ��ȷ�IJ�������Ϊ ������ţ����������̼���Ƶ���������Ϊ ��ֻ���г�����ʽ����