��Ŀ����
CuSO4��Һ����ѧ��ѧ����ũҵ�����г�����һ���Լ���
��1��ijͬѧ����CuSO4��Һʱ�������һ������������Һ�������ӷ���ʽ˵����ԭ����______��
��2����ͬѧ�����Ƶõ�CuSO4��Һ����������ʵ��̽����
��ͼһ�Ǹ��ݷ�ӦZn+CuSO4=Cu+ZnSO4��Ƴɵ�пͭԭ��أ��������Һ����______���ZnSO4����CuSO4������Һ��Cu���ĵ缫��Ӧʽ��______��
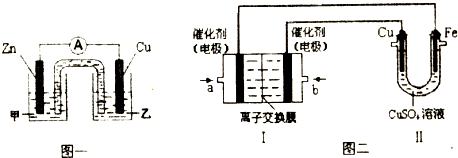
��ͼ���У�I�Ǽ���ȼ�ϵ�أ��������ҺΪKOH��Һ���Ľṹʾ��ͼ����ͬѧ����II��ʵ�����϶�ͭ����b��ͨ�����______���CH4����O2������a���缫�Ϸ����ĵ缫��Ӧʽ��______������II�е缫����Ϊ���Ե缫�����ʱ�Ļ�ѧ��Ӧ����ʽΪ______������II�е缫����Ϊ���Ե缫�����Һ��Ϊ����0.04molCuSO4��0.04molNaCl�Ļ����Һ400mL������������������Ϊ672mL����״���£�ʱ����Һ��pH=______�����������Һ������䣩��
��3����������CuSO4-5H2O������ʯ�Һ�ˮ��һ��������ϣ����ɵõ�������Һ��ɱ������������Ч�ɷ�Ϊ���ܵļ�ʽ����ͭ[xCuSO4-yCu��OH��2]��Ϊ�ⶨij��ʽ����ͭ����ɽ���������ʵ�飺ȡ�������ļ�ʽ����ͭ��Ʒ���ݣ�һ�ݵμ�ϡ������ǡ����ȫ�ܽ⣬��һ�ݸ������պ�ֻ�õ�CuO���壮����������ʾn��HCl����n��CuO��=3��2����ü�ʽ����ͭ�Ļ�ѧʽ��x��y=______��
��1��ijͬѧ����CuSO4��Һʱ�������һ������������Һ�������ӷ���ʽ˵����ԭ����______��
��2����ͬѧ�����Ƶõ�CuSO4��Һ����������ʵ��̽����
��ͼһ�Ǹ��ݷ�ӦZn+CuSO4=Cu+ZnSO4��Ƴɵ�пͭԭ��أ��������Һ����______���ZnSO4����CuSO4������Һ��Cu���ĵ缫��Ӧʽ��______��

��ͼ���У�I�Ǽ���ȼ�ϵ�أ��������ҺΪKOH��Һ���Ľṹʾ��ͼ����ͬѧ����II��ʵ�����϶�ͭ����b��ͨ�����______���CH4����O2������a���缫�Ϸ����ĵ缫��Ӧʽ��______������II�е缫����Ϊ���Ե缫�����ʱ�Ļ�ѧ��Ӧ����ʽΪ______������II�е缫����Ϊ���Ե缫�����Һ��Ϊ����0.04molCuSO4��0.04molNaCl�Ļ����Һ400mL������������������Ϊ672mL����״���£�ʱ����Һ��pH=______�����������Һ������䣩��
��3����������CuSO4-5H2O������ʯ�Һ�ˮ��һ��������ϣ����ɵõ�������Һ��ɱ������������Ч�ɷ�Ϊ���ܵļ�ʽ����ͭ[xCuSO4-yCu��OH��2]��Ϊ�ⶨij��ʽ����ͭ����ɽ���������ʵ�飺ȡ�������ļ�ʽ����ͭ��Ʒ���ݣ�һ�ݵμ�ϡ������ǡ����ȫ�ܽ⣬��һ�ݸ������պ�ֻ�õ�CuO���壮����������ʾn��HCl����n��CuO��=3��2����ü�ʽ����ͭ�Ļ�ѧʽ��x��y=______��
��1��ͭ����ˮ�⣬����������ˮ�⣬ˮ�����ӷ�ӦΪCu2++2H2O

Cu��OH��2+2H+���ʴ�Ϊ��Cu2++2H2O

Cu��OH��2+2H+��
��2���ٸ���ΪZn���������Һ�к�п���ӣ���������Һ��ΪZnSO4��CuΪ�������õ����ӷ�����ԭ��Ӧ���缫��ӦΪCu2++2e-=Cu���ʴ�Ϊ��ZnSO4��Cu2++2e-=Cu��
�����϶�ͭ����CuΪ������Cu�缫��b������bΪԭ��ص���������b��ͨ��O2��a���缫�Ϸ����ĵ缫��Ӧʽ��CH4-8e-+10OH-=CO32-+7H2O��
���Ե缫�������ͭ��Һ�������ᡢCu���������õ�ⷴӦΪ2CuSO4+2H2O
2Cu+O2��+2H2SO4��
II�е缫����Ϊ���Ե缫�����Һ��Ϊ����0.04molCuSO4��0.04molNaCl�Ļ����Һ400mL����������2Cl--2e-=Cl2����4OH--4e-=2H2O+O2������������������Ϊ672mLΪ������������
����������ʵ���Ϊ
=0.03mol����2Cl--2e-=Cl2����֪������Ϊ0.02mol����������Ϊ0.01mol����4OH--4e-=2H2O+O2����4H+��n��H+����=0.04mol��c��H+����=
=0.1mol/L������pH=1��
�ʴ�Ϊ��O2��CH4-8e-+10OH-=CO32-+7H2O��2CuSO4+2H2O
2Cu+O2��+2H2SO4��1��
��3����xCuSO4?yCu��OH��2��yCuCl2��2yHCl����x+y��CuO��
n��HCl����n��CuO��=3��2����2y����x+y��=3��2��
���x��y=1��3���ʴ�Ϊ��1��3��

Cu��OH��2+2H+���ʴ�Ϊ��Cu2++2H2O

Cu��OH��2+2H+��
��2���ٸ���ΪZn���������Һ�к�п���ӣ���������Һ��ΪZnSO4��CuΪ�������õ����ӷ�����ԭ��Ӧ���缫��ӦΪCu2++2e-=Cu���ʴ�Ϊ��ZnSO4��Cu2++2e-=Cu��
�����϶�ͭ����CuΪ������Cu�缫��b������bΪԭ��ص���������b��ͨ��O2��a���缫�Ϸ����ĵ缫��Ӧʽ��CH4-8e-+10OH-=CO32-+7H2O��
���Ե缫�������ͭ��Һ�������ᡢCu���������õ�ⷴӦΪ2CuSO4+2H2O
| ||
II�е缫����Ϊ���Ե缫�����Һ��Ϊ����0.04molCuSO4��0.04molNaCl�Ļ����Һ400mL����������2Cl--2e-=Cl2����4OH--4e-=2H2O+O2������������������Ϊ672mLΪ������������
����������ʵ���Ϊ
| 0.672L |
| 22.4L/mol |
| 0.04mol |
| 0.4L |
�ʴ�Ϊ��O2��CH4-8e-+10OH-=CO32-+7H2O��2CuSO4+2H2O
| ||
��3����xCuSO4?yCu��OH��2��yCuCl2��2yHCl����x+y��CuO��
n��HCl����n��CuO��=3��2����2y����x+y��=3��2��
���x��y=1��3���ʴ�Ϊ��1��3��

��ϰ��ϵ�д�
�����Ŀ

 Cu��OH��2+2H+
Cu��OH��2+2H+


 ������
������