��Ŀ����
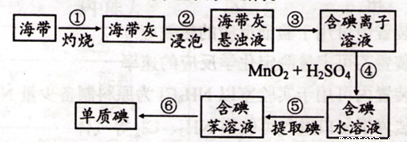
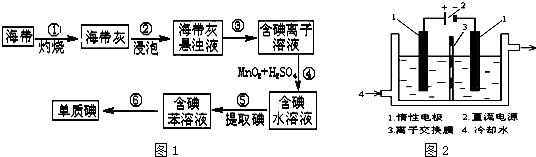
��1��ͼ1Ϊ�����Ƶ������ͼ�������ʵ�����������
��2������أ�KIO3����ʳ�εļӵ����KIO3�����Խ���������H2O2��I�����þ����ɵ��ʵ⣮
��ش��������⣺
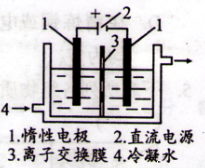
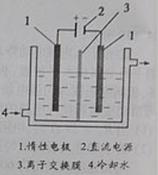
���Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ2��ʾ�����ǰ���Ƚ�һ�����ľ��Ƶ����ڹ�������������Һ���ܽ�ʱ������Ӧ��3I2+6KOH=5KI+KIO3+3H2O��������Һ��������������������������Һ������������
���ʱ��������Ӧ��
�ڵ������У���ͨ�������������Һ���Ƿ���I��������ȷ������Ƿ���ɣ�������жϵ���Ƿ���ɵ�ʵ�鷽�����������±����������ޡ���ѡ���Լ���������Һ��H2O2��Һ��ϡ���ᣮ
| ʵ����� | ʵ�������� |
��2���������������������е����ӡ���������Ӻ����������ӣ��������������ϵ�����ʧ��������IO3-�������������ӷŵ�����������
�����������£������Ӻ͵���������ܷ���������ԭ��Ӧ���ɵⵥ�ʣ������������ɫ��������Һ�Ƿ�����ж��Ƿ��е����ӣ�
�ʴ�Ϊ������MnO2+2I-+4H+=I2+Mn2++2H2O��
��2���������������������е����ӡ���������Ӻ����������ӣ��������������ϵ�����ʧ��������IO3-���缫����ʽΪI-+6OH--6e-=IO3-+3H2O�������������ӷŵ����������������������������ݲ�����
�ʴ�Ϊ��I-+6OH--6e-=IO3-+3H2O�������ݲ�����
�ڵ������Һ�����е�������ӣ����������£������Ӻ͵���������ܷ���������ԭ��Ӧ���ɵⵥ�ʣ�������Һ�������ɫ������ʵ�鷽���ǣ�ȡ�������������Һ���Թ��У���ϡ�����ữ����뼸�ε�����Һ���۲��Ƿ������������������е����ӣ�����ϡ�������еⵥ�����ɣ�������Һ�ͻ����ɫ������ɫ��
�ʴ�Ϊ��
| ʵ����� | ʵ�������� |
| ȡ�������������Һ���Թ��У� ��ϡ�����ữ����뼸�ε�����Һ���۲��Ƿ������ |
�����������˵����I-�������������˵����I-���� |

![]() �ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ������
�ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ������
�����(KIO3)�ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ������
��ˮ������������Խ���������������⻯�����þ����ɵ���
�⡣�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ������ͼ��ʾ��
![]() ��ش��������⣺
��ش��������⣺
(1)���� (����ɫ)�������ʣ�ʵ���ҳ���
�����������ᴿ�����������ʵĹ���⡣
(2)���ǰ���Ƚ�һ�����ľ��Ƶ����ڹ�������������Һ���ܽ�ʱ������Ӧ��
3I2+6KOH=5KI+KIO3+3H2O��������Һ��������������������������Һ������������������ˮ��ȴ��
���ʱ�������Ϸ�����Ӧ�ĵ缫��ӦʽΪ �������Ϲ۲쵽��ʵ�������� ��
(3)�������У�Ϊȷ������Ƿ���ɣ��������Һ���Ƿ���I���������һ��������Һ���Ƿ���I����ʵ�鷽��������Ҫ����д�±���
Ҫ������ҩƷֻ�ܴ������Լ���ѡ��ʵ�������������Ʒ��ѡ��
�Լ���������Һ���⻯�ص�����ֽ������������Һ��ϡ���ᡣ![]()
![]()
![]() w_w w. k#s5_u.c o*m
w_w w. k#s5_u.c o*m
| ʵ�鷽�� | ʵ�������� |
(4)�����ϣ��ӵ��Һ�еõ�����ؾ����ʵ��������£�
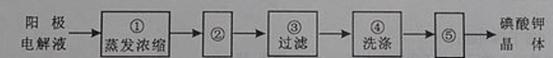
����ڵIJ��������� ������ݵIJ��������� �������ϴ�Ӿ����Ŀ����
��
�ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ������
�����(KIO3)�ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ������
��ˮ������������Խ���������������⻯�����þ����ɵ���
 �⡣�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ������ͼ��ʾ��
�⡣�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ������ͼ��ʾ��
��ش��������⣺
(1)���� (����ɫ)�������ʣ�ʵ���ҳ���
�����������ᴿ�����������ʵĹ���⡣
(2)���ǰ���Ƚ�һ�����ľ��Ƶ����ڹ�������������Һ���ܽ�ʱ������Ӧ��
3I2+6KOH=5KI+KIO3+3H2O��������Һ��������������������������Һ������������������ˮ��ȴ��
���ʱ�������Ϸ�����Ӧ�ĵ缫��ӦʽΪ �������Ϲ۲쵽��ʵ�������� ��
(3)�������У�Ϊȷ������Ƿ���ɣ��������Һ���Ƿ���I���������һ��������Һ���Ƿ���I����ʵ�鷽��������Ҫ����д�±���
Ҫ������ҩƷֻ�ܴ������Լ���ѡ��ʵ�������������Ʒ��ѡ��
�Լ���������Һ���⻯�ص�����ֽ������������Һ��ϡ���ᡣ
| ʵ�鷽�� | ʵ�������� |
|
|
|
(4)�����ϣ��ӵ��Һ�еõ�����ؾ����ʵ��������£�
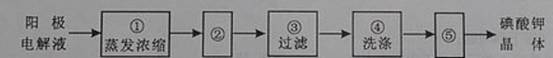
����ڵIJ��������� ������ݵIJ��������� �������ϴ�Ӿ����Ŀ����
��
�ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ������
�����(KIO3)�ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ������
��ˮ������������Խ���������������⻯�����þ����ɵ���
 �⡣�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ������ͼ��ʾ��
�⡣�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ������ͼ��ʾ��
��ش��������⣺
(1)���� (����ɫ)�������ʣ�ʵ���ҳ���
�����������ᴿ�����������ʵĹ���⡣
(2)���ǰ���Ƚ�һ�����ľ��Ƶ����ڹ�������������Һ���ܽ�ʱ������Ӧ��
3I2+6KOH=5KI+KIO3+3H2O��������Һ��������������������������Һ������������������ˮ��ȴ��
���ʱ�������Ϸ�����Ӧ�ĵ缫��ӦʽΪ �������Ϲ۲쵽��ʵ�������� ��
(3)�������У�Ϊȷ������Ƿ���ɣ��������Һ���Ƿ���I���������һ��������Һ���Ƿ���I����ʵ�鷽��������Ҫ����д�±���
Ҫ������ҩƷֻ�ܴ������Լ���ѡ��ʵ�������������Ʒ��ѡ��
�Լ���������Һ���⻯�ص�����ֽ������������Һ��ϡ���ᡣ
|
ʵ�鷽�� |
ʵ�������� |
|
|
|
(4)�����ϣ��ӵ��Һ�еõ�����ؾ����ʵ��������£�
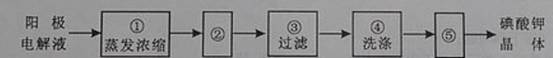
����ڵIJ��������� ������ݵIJ��������� �������ϴ�Ӿ����Ŀ����
��