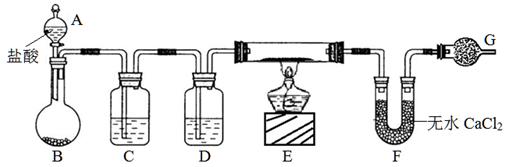
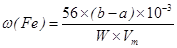��Ŀ����
ij�ռ���Ʒ�����������������õ����ʣ�Ϊ�˵ζ��䴿�ȣ��������µζ�������
A����250mL������ƿ������250mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00mL�ռ���Һ����ƿ�еμӼ��η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷWg�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪC�ı�������Һװ����ʽ�ζ��ܣ�����Һ����¿�ʼ����ΪV1mL
E������ƿ�µ�һ�Ű�ֽ���ζ�����ɫ�պ���ʧΪֹ������ɫ���ٱ仯�����¶���V2mL
�ش��������⣺
��1����ȷ���������˳���ǣ�����ĸ���� �� �� �� D �� ��
��2������E�е���ƿ�µ�һ�Ű�ֽ�������� ��
��3������D��Һ��Ӧ������ �����첿��Ӧ ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Һ��ϴ��ʹ�ⶨ��Ũ�� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����ԭ���� ��
��5��������ζ���ʱ�����в�����ʹ�ⶨ��������Ũ�ȣ�ƫ�͵��� ��
A����ʽ�ζ��ܵ����յ�ʱ�����Ӷ���
B����Һ������ƿ����10 ml����ˮ�ٵζ�
C����ʽ�ζ���������ˮ��ϴ��δ�ò���Һ��ϴ
D����ʽ�ζ���ע����Һ�����������ݣ���ʼ�ζ�
��6�����ռ���Ʒ���ȵļ���ʽΪ ��
A����250mL������ƿ������250mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00mL�ռ���Һ����ƿ�еμӼ��η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷWg�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪC�ı�������Һװ����ʽ�ζ��ܣ�����Һ����¿�ʼ����ΪV1mL
E������ƿ�µ�һ�Ű�ֽ���ζ�����ɫ�պ���ʧΪֹ������ɫ���ٱ仯�����¶���V2mL
�ش��������⣺
��1����ȷ���������˳���ǣ�����ĸ���� �� �� �� D �� ��
��2������E�е���ƿ�µ�һ�Ű�ֽ�������� ��
��3������D��Һ��Ӧ������ �����첿��Ӧ ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Һ��ϴ��ʹ�ⶨ��Ũ�� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����ԭ���� ��
��5��������ζ���ʱ�����в�����ʹ�ⶨ��������Ũ�ȣ�ƫ�͵��� ��
A����ʽ�ζ��ܵ����յ�ʱ�����Ӷ���
B����Һ������ƿ����10 ml����ˮ�ٵζ�
C����ʽ�ζ���������ˮ��ϴ��δ�ò���Һ��ϴ
D����ʽ�ζ���ע����Һ�����������ݣ���ʼ�ζ�
��6�����ռ���Ʒ���ȵļ���ʽΪ ��
��1��C��A��B��E�� ��2�����ڹ۲���Һ��ɫ�ı仯��
��3����0����0������ijһ�̶ȣ�������Һ�������ݣ�
��4��ƫ�ߣ��ô�����������¶����ı�����Һ��
��5��A
��6��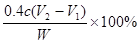
��3����0����0������ijһ�̶ȣ�������Һ�������ݣ�
��4��ƫ�ߣ��ô�����������¶����ı�����Һ��
��5��A
��6��
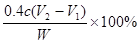
��1�������к͵ζ�ʵ�������
��2����ֽ��ʹ��Һ��ɫ�ı仯�����������ڹ۲���Һ��ɫ�ı仯��
��3���ζ��ܵĿ̶������϶���������ģ�����Һ��Ӧ�õ��ڵ���0����0������ijһ�̶ȡ��ζ��ܵļ��첿��Ӧ���dz�����Һ�������ݡ�
��4����ƿ���ռ���Һ��ϴ������ƿ�ڼ�����ʵ������ӣ��������������ⶨ���ƫ�ߡ�
��5�����Ӷ����������ƫС����������ƫС���ⶨ���ƫ�͡�����Һϡ�ͣ���û�иı�OH�������ʵ��������Բⶨ������䡣û���ñ�Һ��ϴ�����൱��ϡ�����ᣬ���������Ľ�ƫ�ⶨ���ƫ�ߡ������������ݣ���˵����ʼ����ƫС����˲ⶨ���ƫ�ߣ���ѡA��
��6������������ ������ԭ��Ʒ���������Ƶ�������
������ԭ��Ʒ���������Ƶ�������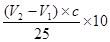 �������ռ���Ʒ���ȵļ���ʽΪ
�������ռ���Ʒ���ȵļ���ʽΪ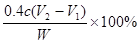 ��
��
��2����ֽ��ʹ��Һ��ɫ�ı仯�����������ڹ۲���Һ��ɫ�ı仯��
��3���ζ��ܵĿ̶������϶���������ģ�����Һ��Ӧ�õ��ڵ���0����0������ijһ�̶ȡ��ζ��ܵļ��첿��Ӧ���dz�����Һ�������ݡ�
��4����ƿ���ռ���Һ��ϴ������ƿ�ڼ�����ʵ������ӣ��������������ⶨ���ƫ�ߡ�
��5�����Ӷ����������ƫС����������ƫС���ⶨ���ƫ�͡�����Һϡ�ͣ���û�иı�OH�������ʵ��������Բⶨ������䡣û���ñ�Һ��ϴ�����൱��ϡ�����ᣬ���������Ľ�ƫ�ⶨ���ƫ�ߡ������������ݣ���˵����ʼ����ƫС����˲ⶨ���ƫ�ߣ���ѡA��
��6������������
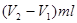 ������ԭ��Ʒ���������Ƶ�������
������ԭ��Ʒ���������Ƶ�������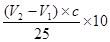 �������ռ���Ʒ���ȵļ���ʽΪ
�������ռ���Ʒ���ȵļ���ʽΪ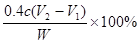 ��
��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

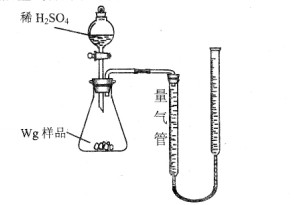
 (Fe) (b-a)��10-3L
(Fe) (b-a)��10-3L