��Ŀ����
��98%���ܶ�Ϊ1.84g/cm3����Ũ��������3mol/L��ϡ����100ml���ش��������⣺
��1����Ҫ����Ͳ��ȡŨ����______ml��
��2�����Ʋ����ɷֽ�����¼�������ȷ�IJ���˳����______
A�����ݼ��㣬����Ͳȡһ�������Ũ����
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
E����������ƿ���ӣ���ҡ��
F���ý�ͷ�ιܼ�����ˮ��ʹ��Һ��Һ��ǡ����̶�������
G������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���1-2cm
��3�����ڴ��������ʹ�Ƶõ�ϡ������ҺŨ��ƫС����______����д��ţ���
A������Ͳ��ȡһ��Һ��ʱ������Һ�����
B��ʹ������ƿ������Һʱ������Һ�涨�ݺ�������ҺŨ��
C��û��������ˮϴ���ձ�2��3�Σ�������Һ��������ƿ��
D������ƿ��������ˮϴ����û�к��
E������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ�����������ˮʹҺ�氼����̶�������
F�������ƺõ���Һ�����������ˮϴ�����Լ�ƿ�б��ã�
�⣺��1��98%���ܶ�Ϊ1.84g/cm3����Ũ��������ʵ���Ũ��Ϊ mol/L=18.4mol/L
mol/L=18.4mol/L
����ҪŨ��������ΪV������ϡ�Ͷ��ɣ���Һϡ��ǰ�����ʵ����ʵ������䣬��
V��18.4mol/L=3mol/L��100ml
���V=16.3ml��
�ʴ�Ϊ��16.3��
��2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������20mL��Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ�������������ϴ��Һע������ƿ�����ظ�����2-3�Σ�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ���ˮ����Һ��Һ��ǡ����̶������У���������ƿ���ӣ���ҡ�ȣ�ʵ�����˳��������ADCBGFE��
�ʴ�Ϊ��ADCBGFE��
��3��A������Ͳ��ȡһ��Һ��ʱ������Һ���������ȡŨ��������ƫС��������ҺŨ��ƫС����A���ϣ�
B��ʹ������ƿ������Һʱ������Һ�涨�ݣ���Һ���ƫС��������ҺŨ��ƫ��B�����ϣ�
C��û��������ˮϴ���ձ�2��3�Σ�������Һ��������ƿ�У���������ƿ����������ʵ���ƫС��������ҺŨ��ƫС����C���ϣ�
D���������ˮ���ݣ�����ƿ��������ˮϴ����û�к�ɣ���������ҺŨ����Ӱ�죬��D�����ϣ�
E������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ���Һ���ƫ��������ҺŨ��ƫС����ʹ��������ˮʹҺ�氼����̶������У�������ҺŨ����ƫС����E���ϣ�
F�������ƺõ���Һ�����������ˮϴ�����Լ�ƿ�б��ã���Һ��ϡ�ͣ�Ũ��ƫС����F���ϣ�
��ѡ��ACEF��
��������1������c=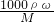 ����Ũ�����Ũ�ȣ��ٸ���ϡ�Ͷ��ɣ���Һϡ��ǰ�����ʵ����ʵ����������Ũ����������
����Ũ�����Ũ�ȣ��ٸ���ϡ�Ͷ��ɣ���Һϡ��ǰ�����ʵ����ʵ����������Ũ����������
��2������ʵ�����������в���˳������
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c= �ж϶�������ҺŨ��Ӱ�죮
�ж϶�������ҺŨ��Ӱ�죮
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c= �з������������ʵ����ʵ��������Һ�������Ӱ���ж�
�з������������ʵ����ʵ��������Һ�������Ӱ���ж�
 mol/L=18.4mol/L
mol/L=18.4mol/L����ҪŨ��������ΪV������ϡ�Ͷ��ɣ���Һϡ��ǰ�����ʵ����ʵ������䣬��
V��18.4mol/L=3mol/L��100ml
���V=16.3ml��
�ʴ�Ϊ��16.3��
��2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������20mL��Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ�������������ϴ��Һע������ƿ�����ظ�����2-3�Σ�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ���ˮ����Һ��Һ��ǡ����̶������У���������ƿ���ӣ���ҡ�ȣ�ʵ�����˳��������ADCBGFE��
�ʴ�Ϊ��ADCBGFE��
��3��A������Ͳ��ȡһ��Һ��ʱ������Һ���������ȡŨ��������ƫС��������ҺŨ��ƫС����A���ϣ�
B��ʹ������ƿ������Һʱ������Һ�涨�ݣ���Һ���ƫС��������ҺŨ��ƫ��B�����ϣ�
C��û��������ˮϴ���ձ�2��3�Σ�������Һ��������ƿ�У���������ƿ����������ʵ���ƫС��������ҺŨ��ƫС����C���ϣ�
D���������ˮ���ݣ�����ƿ��������ˮϴ����û�к�ɣ���������ҺŨ����Ӱ�죬��D�����ϣ�
E������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ���Һ���ƫ��������ҺŨ��ƫС����ʹ��������ˮʹҺ�氼����̶������У�������ҺŨ����ƫС����E���ϣ�
F�������ƺõ���Һ�����������ˮϴ�����Լ�ƿ�б��ã���Һ��ϡ�ͣ�Ũ��ƫС����F���ϣ�
��ѡ��ACEF��
��������1������c=
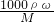 ����Ũ�����Ũ�ȣ��ٸ���ϡ�Ͷ��ɣ���Һϡ��ǰ�����ʵ����ʵ����������Ũ����������
����Ũ�����Ũ�ȣ��ٸ���ϡ�Ͷ��ɣ���Һϡ��ǰ�����ʵ����ʵ����������Ũ������������2������ʵ�����������в���˳������
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
 �ж϶�������ҺŨ��Ӱ�죮
�ж϶�������ҺŨ��Ӱ�죮���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
 �з������������ʵ����ʵ��������Һ�������Ӱ���ж�
�з������������ʵ����ʵ��������Һ�������Ӱ���ж� 
��ϰ��ϵ�д�
�����Ŀ
