��Ŀ����
��2012?����һģ��W��X��Y��Z��N�Ƕ�����Ԫ�أ����ǵĺ˵������������
��1��W���ʵĻ�ѧʽΪ
��2��W��X�γɵĻ������еĹ��ۼ���ԭ�ӹ�����ص���ʽ����
��3��Y��Z��N����Ԫ�ص�һ�������ɴ�С��˳��Ϊ
��4��Z��X�γɵĻ����ﹹ�ɵľ�������
��5��Y����Ķѻ���ʽ����ͼ��ʾ��������Yԭ�ӵ���λ��Ϊ
��6�����õ��Y��N���ڻ�����ķ�������Y���ʵ�ԭ����
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| W | ԭ�Ӻ��������� |
| X | ԭ�Ӻ���s�Dz��ϵĵ���������p�Dz��ϵĵ���������� |
| Y | Ԫ�ص����Ӱ뾶�ڸ���������С |
| Z | ԭ�Ӻ���p�Dz��ϵĵ���������s�Dz��ϵĵ���������2 |
| N | �����������ȴ�����������1 |
H2
H2
��ZԪ��ԭ�Ӻ�����8
8
��ԭ�ӹ������˵��ӣ���2��W��X�γɵĻ������еĹ��ۼ���ԭ�ӹ�����ص���ʽ����
��
��
������3��Y��Z��N����Ԫ�ص�һ�������ɴ�С��˳��Ϊ
Cl��Si��Al
Cl��Si��Al
����Ԫ�ط��ţ�����4��Z��X�γɵĻ����ﹹ�ɵľ�������
ԭ��
ԭ��
���壮
��5��Y����Ķѻ���ʽ����ͼ��ʾ��������Yԭ�ӵ���λ��Ϊ
12
12
����6�����õ��Y��N���ڻ�����ķ�������Y���ʵ�ԭ����
AlCl3�ۻ��������ƶ�������
AlCl3�ۻ��������ƶ�������
��������Wԭ�Ӻ��������ӣ�ӦΪHԪ�أ�
Xԭ�Ӻ���s�Dz��ϵĵ���������p�Dz��ϵĵ���������ȣ�ӦΪOԪ�أ�
YԪ�ص����Ӱ뾶�ڸ���������С��ӦΪAlԪ�أ�
Zԭ�Ӻ���p�Dz��ϵĵ���������s�Dz��ϵĵ���������2��ӦΪSiԪ�أ�
��������Ų�Ϊ1s22s22p63s23p2��N�����������ȴ�����������1��ӦΪClԪ�أ�
��϶�Ӧ���ʡ�������������Լ���ĿҪ��ɽ����⣮
Xԭ�Ӻ���s�Dz��ϵĵ���������p�Dz��ϵĵ���������ȣ�ӦΪOԪ�أ�
YԪ�ص����Ӱ뾶�ڸ���������С��ӦΪAlԪ�أ�
Zԭ�Ӻ���p�Dz��ϵĵ���������s�Dz��ϵĵ���������2��ӦΪSiԪ�أ�
��������Ų�Ϊ1s22s22p63s23p2��N�����������ȴ�����������1��ӦΪClԪ�أ�
��϶�Ӧ���ʡ�������������Լ���ĿҪ��ɽ����⣮
����⣺Wԭ�Ӻ��������ӣ�ӦΪHԪ�أ�
Xԭ�Ӻ���s�Dz��ϵĵ���������p�Dz��ϵĵ���������ȣ�ӦΪOԪ�أ�
YԪ�ص����Ӱ뾶�ڸ���������С��ӦΪAlԪ�أ�
Zԭ�Ӻ���p�Dz��ϵĵ���������s�Dz��ϵĵ���������2��ӦΪSiԪ�أ�
��������Ų�Ϊ1s22s22p63s23p2��N�����������ȴ�����������1��ӦΪClԪ�أ�
��1�������Ϸ�����֪W���ʵĻ�ѧʽΪH2��ZΪSiԪ�أ���������Ų�Ϊ1s22s22p63s23p2��ԭ�Ӻ�����8��ԭ�ӹ������˵��ӣ�
�ʴ�Ϊ��H2��8��
��2��WΪH��XΪO���������еĹ��ۼ���ԭ�ӹ�����ص���ʽ���ڦļ���
�ʴ�Ϊ���ģ�
��3��Y��Z��N����Ԫ�طֱ�ΪAl��Si��Cl������Ԫ�ص�һ�������ɴ�С��˳��ΪCl��Si��Al��
�ʴ�Ϊ��Cl��Si��Al��
��4��XΪO��ZΪSi�����ɵĻ�����Ϊԭ�Ӿ��壬
�ʴ�Ϊ��ԭ�ӣ�
��5��YΪAl��Ϊ���������ѻ��ṹ��Al����ͭ�����������������ֽṹ��ԭ�ӵ���λ����12��
�ʴ�Ϊ��12��
��6��Y��N�۵Ļ�����Ϊ�Ȼ�����Ϊ���ۻ����AlCl3�ۻ��������ƶ������ӣ����ܵ��磬
�ʴ�Ϊ��AlCl3�ۻ��������ƶ������ӣ�
Xԭ�Ӻ���s�Dz��ϵĵ���������p�Dz��ϵĵ���������ȣ�ӦΪOԪ�أ�
YԪ�ص����Ӱ뾶�ڸ���������С��ӦΪAlԪ�أ�
Zԭ�Ӻ���p�Dz��ϵĵ���������s�Dz��ϵĵ���������2��ӦΪSiԪ�أ�
��������Ų�Ϊ1s22s22p63s23p2��N�����������ȴ�����������1��ӦΪClԪ�أ�
��1�������Ϸ�����֪W���ʵĻ�ѧʽΪH2��ZΪSiԪ�أ���������Ų�Ϊ1s22s22p63s23p2��ԭ�Ӻ�����8��ԭ�ӹ������˵��ӣ�
�ʴ�Ϊ��H2��8��
��2��WΪH��XΪO���������еĹ��ۼ���ԭ�ӹ�����ص���ʽ���ڦļ���
�ʴ�Ϊ���ģ�
��3��Y��Z��N����Ԫ�طֱ�ΪAl��Si��Cl������Ԫ�ص�һ�������ɴ�С��˳��ΪCl��Si��Al��
�ʴ�Ϊ��Cl��Si��Al��
��4��XΪO��ZΪSi�����ɵĻ�����Ϊԭ�Ӿ��壬
�ʴ�Ϊ��ԭ�ӣ�
��5��YΪAl��Ϊ���������ѻ��ṹ��Al����ͭ�����������������ֽṹ��ԭ�ӵ���λ����12��
�ʴ�Ϊ��12��
��6��Y��N�۵Ļ�����Ϊ�Ȼ�����Ϊ���ۻ����AlCl3�ۻ��������ƶ������ӣ����ܵ��磬
�ʴ�Ϊ��AlCl3�ۻ��������ƶ������ӣ�
�����������ۺϿ���ԭ�ӽṹ��λ�ú����ʵĹ�ϵ��Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬ע�����ԭ�ӵĺ�������Ų���������ȷԪ�ص�����Ϊ������Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
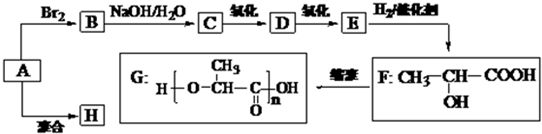



 ��2012?����һģ����pH��ͬ������������Һ¶���ڿ�����һ��ʱ�����Һ��pH��ʱ��ı仯�����ͼ��ʾ����a��b��c������Һ�ֱ����Ϊ��������
��2012?����һģ����pH��ͬ������������Һ¶���ڿ�����һ��ʱ�����Һ��pH��ʱ��ı仯�����ͼ��ʾ����a��b��c������Һ�ֱ����Ϊ�������� ��2012?����һģ���Ҵ�ȼ�ϵ���в��û����������ܼ�������ܷ�ӦΪ��C2H5OH+3O2=2CO2+3H2O����˵��������ǣ�������
��2012?����һģ���Ҵ�ȼ�ϵ���в��û����������ܼ�������ܷ�ӦΪ��C2H5OH+3O2=2CO2+3H2O����˵��������ǣ�������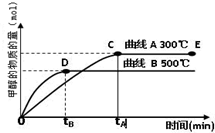 ��2012?����һģ����Դ����������������ٵ��ش���⣬�ձ����������ĺ�й©�¹����������ǶԺ���Դ�Ŀֻţ����״���δ����Ҫ����ɫ��Դ֮һ��
��2012?����һģ����Դ����������������ٵ��ش���⣬�ձ����������ĺ�й©�¹����������ǶԺ���Դ�Ŀֻţ����״���δ����Ҫ����ɫ��Դ֮һ��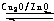 CH3OH��g�����״������ʵ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ��
CH3OH��g�����״������ʵ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ��