��Ŀ����
��8�֣�ijͬѧΪ��̽���Ȼ�淋����ʣ�����������ʵ�飬���㰴Ҫ��ش��������⡣
��1������100mL1mol/L��NH4Cl��Һ����ͬѧӦ��������ƽ����NH4Cl���������Ϊ g��
�������������ձ�����ͷ�ιܡ��������Ȳ���������
�ٻ�ȱ�ٵ������� ��
��ʹ������ƿǰ������е�һ�������� ��
��2����ͬѧ������ͼ��ʾ��װ�������йذ���������ʵ�顣
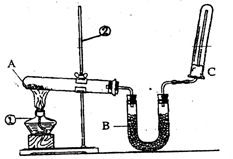
��д��ʵ�����ư����Ļ�ѧ����ʽ�� ��
��д��B��ʢ�ŵ��Ǽ�ʯ���������� ��
�۰�����������ˮ���ڰ�ˮ�еμӷ�̪������ ��
�ܼ��鰱���ķ����� ��
��6�֣���ϡ�����з���������ͭƬ��
��1����Ӧ�Ļ�ѧ����ʽΪ ��
��2������Ӧֹͣ���ټ�������25%��ϡ���ᣬ��ʱͭƬ���������ݲ�������ԭ���� ���������ӷ���ʽ��ʾ��
��3������12.8gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ������������5.6L����
״���£����������ĵ���������ʵ����� ��
��1������100mL1mol/L��NH4Cl��Һ����ͬѧӦ��������ƽ����NH4Cl���������Ϊ g��
�������������ձ�����ͷ�ιܡ��������Ȳ���������
�ٻ�ȱ�ٵ������� ��
��ʹ������ƿǰ������е�һ�������� ��
��2����ͬѧ������ͼ��ʾ��װ�������йذ���������ʵ�顣

��д��ʵ�����ư����Ļ�ѧ����ʽ�� ��
��д��B��ʢ�ŵ��Ǽ�ʯ���������� ��
�۰�����������ˮ���ڰ�ˮ�еμӷ�̪������ ��
�ܼ��鰱���ķ����� ��
��6�֣���ϡ�����з���������ͭƬ��
��1����Ӧ�Ļ�ѧ����ʽΪ ��
��2������Ӧֹͣ���ټ�������25%��ϡ���ᣬ��ʱͭƬ���������ݲ�������ԭ���� ���������ӷ���ʽ��ʾ��
��3������12.8gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ������������5.6L����
״���£����������ĵ���������ʵ����� ��
��8�֣���1��5.4g����100mL����ƿ �ڼ���Ƿ�©ˮ
��2���٢�Ca(OH)2 + NH4Cl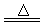 CaCl2 + 2NH3�� + 2H2O
CaCl2 + 2NH3�� + 2H2O
������ˮ���� ����Һ����ɫ��Ϊ��ɫ
����ʪ��ĺ�ɫʯ����ֽ��պ��Ũ����IJ��������������ɸ���)
��6�֣�ÿ��2�֣�
��1��3Cu + 8HNO3��ϡ��= 3Cu(NO3)2 + 2NO�� + 4H2O
��2��3Cu + 8H+ + 2NO3-= 3Cu2+ + 2NO�� + 4H2O
��3��0.65mol
��2���٢�Ca(OH)2 + NH4Cl
 CaCl2 + 2NH3�� + 2H2O
CaCl2 + 2NH3�� + 2H2O������ˮ���� ����Һ����ɫ��Ϊ��ɫ
����ʪ��ĺ�ɫʯ����ֽ��պ��Ũ����IJ��������������ɸ���)
��6�֣�ÿ��2�֣�
��1��3Cu + 8HNO3��ϡ��= 3Cu(NO3)2 + 2NO�� + 4H2O
��2��3Cu + 8H+ + 2NO3-= 3Cu2+ + 2NO�� + 4H2O
��3��0.65mol
��1��100mL1mol/L��NH4Cl��Һ�����ʵ����ʵ�����0.1L��1.0mol/L��0.1mol��������0.1mol��53.5g/mol��5.35g����������������ƽ��������������5.4g��
�ٸ�������������֪����ȱ��100mL����ƿ��
������ƿ��ʹ��֮ǰ���������Ƿ�©ˮ��
��2����ʵ������ȡ�����ķ���ʽΪCa(OH)2 + NH4Cl CaCl2 + 2NH3�� + 2H2O��
CaCl2 + 2NH3�� + 2H2O��
�ڰ����Ǽ������壬���Ը��ﰱ����Ҫ�ü�ʯ�ҡ�
�۰�������ˮ������һˮ�ϰ�����Һ�Լ��ԣ�������Һ����ɫ��Ϊ��ɫ��
�����ð����Ǽ���������м��顣���Լ��鰱��������ʪ��ĺ�ɫʯ����ֽ��պ��Ũ����IJ�������
��1���������ǿ�����ԣ��ܰ�ͭ��������ӦʽΪ3Cu + 8HNO3��ϡ��= 3Cu(NO3)2 + 2NO�� + 4H2O��
��2���ڷ�Ӧ��������ͭ���ɡ�����������Һ�У������ξ��������ԣ����Լ�������ͭƬ�����ӷ���ʽΪ3Cu + 8H+ + 2NO3-= 3Cu2+ + 2NO�� + 4H2O��
��3��5.6LNO�ڱ�״���µ����ʵ�����0.25mol����ԭ��������0.25mol��ͭ��12.8g�����ʵ�����0.2mol������������ͭҲ��0.2mol������û�б���ԭ��������0.4mol����˲μӷ�Ӧ��������0.65mol��
�ٸ�������������֪����ȱ��100mL����ƿ��
������ƿ��ʹ��֮ǰ���������Ƿ�©ˮ��
��2����ʵ������ȡ�����ķ���ʽΪCa(OH)2 + NH4Cl
 CaCl2 + 2NH3�� + 2H2O��
CaCl2 + 2NH3�� + 2H2O���ڰ����Ǽ������壬���Ը��ﰱ����Ҫ�ü�ʯ�ҡ�
�۰�������ˮ������һˮ�ϰ�����Һ�Լ��ԣ�������Һ����ɫ��Ϊ��ɫ��
�����ð����Ǽ���������м��顣���Լ��鰱��������ʪ��ĺ�ɫʯ����ֽ��պ��Ũ����IJ�������
��1���������ǿ�����ԣ��ܰ�ͭ��������ӦʽΪ3Cu + 8HNO3��ϡ��= 3Cu(NO3)2 + 2NO�� + 4H2O��
��2���ڷ�Ӧ��������ͭ���ɡ�����������Һ�У������ξ��������ԣ����Լ�������ͭƬ�����ӷ���ʽΪ3Cu + 8H+ + 2NO3-= 3Cu2+ + 2NO�� + 4H2O��
��3��5.6LNO�ڱ�״���µ����ʵ�����0.25mol����ԭ��������0.25mol��ͭ��12.8g�����ʵ�����0.2mol������������ͭҲ��0.2mol������û�б���ԭ��������0.4mol����˲μӷ�Ӧ��������0.65mol��

��ϰ��ϵ�д�
�����Ŀ