��Ŀ����
���и���Һ�У��������ʵ���Ũ�ȹ�ϵ��ȷ����
| A��0.1 mol��L��1 Na2CO3��Һ��c(Na+)=2c(HCO3��)+2c(CO32��)+2c(H2CO3) |
| B��0.1 mol��L��1 NH4Cl��Һ��c(NH4+)=c(Cl��) |
| C�����������Һ�м����������ᣬ�õ������Ի����Һ��c(Na+) > c(CH3COO��) > c(H+) > c(OH��) |
| D�������£���pH=2�������pH=12�İ�ˮ�������Ϻ�c(NH4+) > c(Cl��) > c(OH��) > c(H+) |
AD
���������A��Na2CO3��Һ�У����ݻ�ѧʽ�ɵ������غ��ʽc(Na+)=2c(HCO3��)+2c(CO32��)+2c(H2CO3)����ȷ��B��NH4Cl��Һ��笠�����ˮ��ʹc(NH4+)<c(Cl��)������C����������Һ�м����������ᣬ�õ������Ի����Һ��˵���������̶ȴ��ڴ��������ˮ��̶ȣ�����c(CH3COO��) > c(Na+)������D����ˮ��������Һ������ˮ��Ũ�ȴ��������Ũ�ȣ��������Ϻ�ˮ��������Һ�ʼ��ԣ�����c(NH4+) > c(Cl��) > c(OH��) > c(H+)����ȷ����ѡAD��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
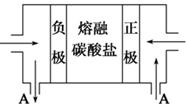
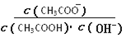 ����
���� c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)
c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)

