��Ŀ����
ijϡ�����ϡ����Ļ����Һ200 mL��ƽ���ֳ����ݣ�������һ��������ͭ�ۣ�������ܽ�19.2 g(��֪����ֻ�ܱ���ԭΪNO����)������һ�����������ۣ�������������������������ӵı仯��ͼ��ʾ�����з��������������( )
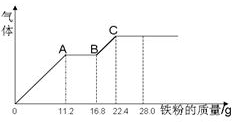

| A��ԭ���Һ��NO3-�����ʵ���Ϊ0��4mol |
| B��c(H2SO4)Ϊ5mol��L-1 |
| C����Һ����������ΪFeSO4 |
| D��OA�β�����NO��AB�η�ӦΪ2Fe3++Fe = 3Fe2+��BC�β������� |
B
���������ͭ��������Һ��Ӧ�����ӷ���ʽ�ǣ�3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��n(Cu)=19��2g��64g/mol=0��3mol,����n(NO3-)=0��2mol����ԭ��Һ�к���������ӵ����ʵ�����0��4mol����ȷ��������һ����Һ��˵�������ķ�Ӧ�ǣ�O��A�Σ�Fe+4H++NO3-=Fe3++NO��+2H2O;A��B�Σ�2Fe3++Fe=3Fe2+;B��C�Σ�Fe+2H+= Fe2++H2������Ӧ�������ҺΪFeSO4��Һ����O��A��n(Fe)=0��2mol,���ĵ�������n(H+)=0��8mol,��B��C��n(Fe)=0��1mol,���ĵ�������n(H+)=0��2mol����n(H+)��=0��8mol+0��2mol=1mol������n(HNO3)=n(NO3-)=0��2mol,����n(H2SO4)=(1��0-0��2)mol/2=0��4mol����C(H2SO4)=0��4mol��0��1L=4mol/L�����Դ���ѡ��Ϊ���¡�

��ϰ��ϵ�д�
�����Ŀ
 ��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�
��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�