��Ŀ����
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
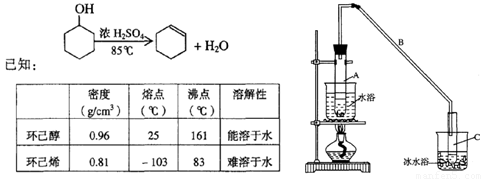
��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ������B���˵�������е������� ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ�� ��(���ϻ���)����Һ����_________ �������ţ���ѡ��ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ��룬Ŀ����_____________________________ ��

���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� ����ѡ��
a������ʱ��70�濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
(1) �ٷ����� ���� �ڷ�ֹ����ϩ�ӷ�
(2) ���ϲ� C ��g ��ȴˮ�������γ����� ��83�� c
��������

ij��ѧС���������������������װ�ã���ͼ��)�Ի������Ʊ�����ϩ��

��֪��
| �ܶȣ�g��cm-3) | �۵㣨��)�� | �㣨��) | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |

ͼ��
(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������_____________������B���˵�������е�������_______________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_________________________________________________��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ��кͱ���ʳ��ˮ�������á��ֲ㣬����ϩ��_______________�㣨��ϡ����¡�������Һ����_______________�������ţ�ϴ�ӡ�
a.KMnO4��Һ
b.ϡH2SO4
c.Na2CO3��Һ

ͼ��
���ٽ�����ϩ��ͼ��װ��������ȴˮ��____________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����____________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��____________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________________________��
a.����ʱ��70 �濪ʼ�ռ���Ʒ
b.������ʵ����������
c.�Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������____________��
a.�ø������������Һ b.�ý����� c.�ⶨ�е�
(11��)ij��ѧС���������������������װ��(��ͼ)���Ի������Ʊ�����ϩ��
��֪��

| | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ������B���˵�������е������� ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ�� ��(���ϻ���)����Һ���� (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ��ͼװ��������ȴˮ�� (������)�ڽ��롣����ʱҪ������ʯ�ҵ�Ŀ�� ��

���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� ���ң�ʵ���ƵõĻ�
��ϩ��Ʒ�����������۲��������ܵ�ԭ���� ��
a������ʱ��70�濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������� ��
a�������Ը��������Һ b���ý����� c���ⶨ�е�




