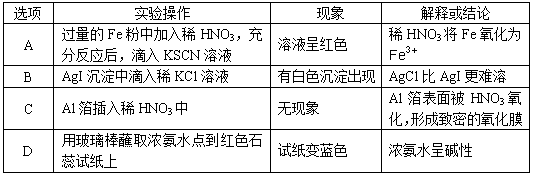
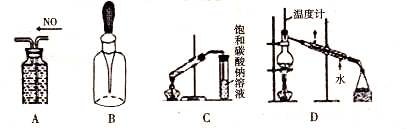
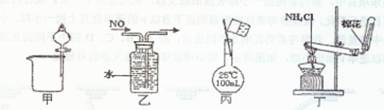
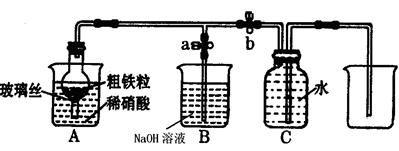
��Ŀ����
����16�֣���1������ͭ������ܽ������ܽ���̽�����ʵ���ҳ�����ˮ�����Լӿ��ܽ����ʣ���������������ǣ����Ҫ˵��ԭ��______________________����β�������ˮ���Ƴ�����Ľ�Ũ��CuSO4��Һ____________________��
��2��ϡNa2S��Һ��һ�ֳ�������ζ������AlCl3��Һ��������ζ�Ӿ磬�����ӷ���ʽ��ʾ��ζ�Ӿ�����������Ļ�ѧ��Ӧ______________________________________________
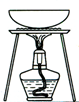
��ij�ռ���Ʒ�к����������������õĿ��������ʣ�Ϊ�˲ⶨ�䴿�ȣ��������µζ�������
E������ƿ�µ�һ�Ű�ֽ���ζ����յ㣬��¼�յ�����������V2 mL��
�ش��������⣺
(1)��ȷ�IJ��������˳����(��д��ĸ)
________��________��________��________��________��
(2)�յ㵽���������________________________��
(3)����ʽ�ζ���û���ñ�H2SO4��ϴ���Բⶨ���Ӱ��________���ζ�ǰ���Ӷ����ζ����Ӷ����Բⶨ���Ӱ��________ ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족��������������ȷ)��
(4)���ռ�İٷֺ�����________��
��2��ϡNa2S��Һ��һ�ֳ�������ζ������AlCl3��Һ��������ζ�Ӿ磬�����ӷ���ʽ��ʾ��ζ�Ӿ�����������Ļ�ѧ��Ӧ______________________________________________
��ij�ռ���Ʒ�к����������������õĿ��������ʣ�Ϊ�˲ⶨ�䴿�ȣ��������µζ�������
| A������Һת����250 mL����ƿ�У���ˮ���̶��ߣ� |
| B������Һ��(���ʽ�ζ���)��ȡ25.00 mL�ռ���Һ����ƿ�в��Ӽ��μ�����ָʾ���� |
| C������ƽ��ȷ��ȡ�ռ���Ʒw g�����ձ��м�����ˮ�ܽ⣻ |
| D�������ʵ���Ũ��Ϊm mol?L��1�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶���ΪV1 mL�� |
�ش��������⣺
(1)��ȷ�IJ��������˳����(��д��ĸ)
________��________��________��________��________��
(2)�յ㵽���������________________________��
(3)����ʽ�ζ���û���ñ�H2SO4��ϴ���Բⶨ���Ӱ��________���ζ�ǰ���Ӷ����ζ����Ӷ����Բⶨ���Ӱ��________ ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족��������������ȷ)��
(4)���ռ�İٷֺ�����________��
.I��(1)Cu2++2H2O  Cu(OH)2+2H+ ���ȴٽ�����ͭˮ�⣻������ͭ���ܽ���ϡ�����У�Ȼ��������ˮϡ�͡� ��2��2Al3++3S2- +6H2O=2Al(OH)3��+ 3H2S��
Cu(OH)2+2H+ ���ȴٽ�����ͭˮ�⣻������ͭ���ܽ���ϡ�����У�Ȼ��������ˮϡ�͡� ��2��2Al3++3S2- +6H2O=2Al(OH)3��+ 3H2S��
II��(1) CABDE (2)��Һ�л�ɫ��Ϊ��ɫ����30S���ָ�Ϊԭ������ɫ��
��3��ƫ�ߣ�ƫ�� ��4��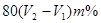
 Cu(OH)2+2H+ ���ȴٽ�����ͭˮ�⣻������ͭ���ܽ���ϡ�����У�Ȼ��������ˮϡ�͡� ��2��2Al3++3S2- +6H2O=2Al(OH)3��+ 3H2S��
Cu(OH)2+2H+ ���ȴٽ�����ͭˮ�⣻������ͭ���ܽ���ϡ�����У�Ȼ��������ˮϡ�͡� ��2��2Al3++3S2- +6H2O=2Al(OH)3��+ 3H2S��II��(1) CABDE (2)��Һ�л�ɫ��Ϊ��ɫ����30S���ָ�Ϊԭ������ɫ��
��3��ƫ�ߣ�ƫ�� ��4��
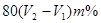
I����1������ͭ��ǿ�������Σ�ˮ������������ͭ��ˮ�������ȵģ����ȴٽ�ˮ�⣬���Ի�������ǡ����Ի�����ˮ�⣬���Խ�����ͭ���ܽ���ϡ�����У�Ȼ��������ˮϡ�͡�
��2������ˮ���Լ��ԣ��Ȼ���ˮ�������ԣ�������ٽ�������ʽΪ2Al3++3S2- +6H2O=2Al(OH)3��+ 3H2S����
II��(1)����ζ�ʵ���������ȷ��˳����CABDE��
��2������ʹ�����Ի�ɫ�������յ�ʱ����������Һ�л�ɫ��Ϊ��ɫ����30S���ָ�Ϊԭ������ɫ��
��3����ʽ�ζ���û���ñ�H2SO4��ϴ�����൱��ϡ�����ᣬ��������������ƫ�࣬�ⶨ���ƫ�ߡ��ζ�ǰ���Ӷ����������ƫ�ζ����Ӷ����������ƫС������������������ƫС���ⶨ���ƫ�͡�
��4���������������ǣ�V2��V1��mL������25m��Һ���������Ƶ����ʵ�����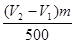 mol�������ܵ�����������
mol�������ܵ�����������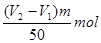 ������ռ�İٷֺ�����
������ռ�İٷֺ�����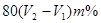 ��
��
��2������ˮ���Լ��ԣ��Ȼ���ˮ�������ԣ�������ٽ�������ʽΪ2Al3++3S2- +6H2O=2Al(OH)3��+ 3H2S����
II��(1)����ζ�ʵ���������ȷ��˳����CABDE��
��2������ʹ�����Ի�ɫ�������յ�ʱ����������Һ�л�ɫ��Ϊ��ɫ����30S���ָ�Ϊԭ������ɫ��
��3����ʽ�ζ���û���ñ�H2SO4��ϴ�����൱��ϡ�����ᣬ��������������ƫ�࣬�ⶨ���ƫ�ߡ��ζ�ǰ���Ӷ����������ƫ�ζ����Ӷ����������ƫС������������������ƫС���ⶨ���ƫ�͡�
��4���������������ǣ�V2��V1��mL������25m��Һ���������Ƶ����ʵ�����
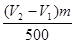 mol�������ܵ�����������
mol�������ܵ�����������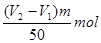 ������ռ�İٷֺ�����
������ռ�İٷֺ�����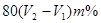 ��
��
��ϰ��ϵ�д�
�����Ŀ