��Ŀ����
ijѧ����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�
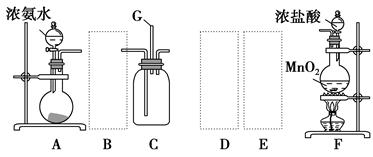
��ش��������⣺
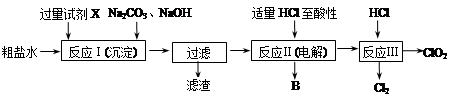
(1)װ��F�з�����Ӧ�����ӷ���ʽ�� ��
(2)װ��A�е���ƿ�ڹ����ѡ�� (ѡ������ѡ��Ĵ���)��
A����ʯ��
B����ʯ��
C����������
D������������
E���ռ�
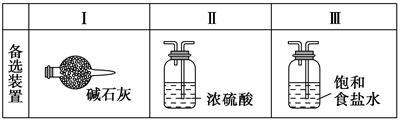
(3)���߿���Ӧ���ӱ�Ҫ�ij���װ�ã������ͼ�ı�ѡװ����ѡ��������������пո�B ��D ��E ��(������)
(4)�����Ͱ����ڳ��������ͻᷴӦ�����Ȼ�狀͵�����װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ�����ʵ�鷽�������ù�������Ȼ�泥� ��
(5)��װ��C�ij����ܿڴ��ݳ���β�����ܺ�����Ⱦ���������壬��δ����� ��


��ش��������⣺
(1)װ��F�з�����Ӧ�����ӷ���ʽ�� ��
(2)װ��A�е���ƿ�ڹ����ѡ�� (ѡ������ѡ��Ĵ���)��
A����ʯ��
B����ʯ��
C����������
D������������
E���ռ�
(3)���߿���Ӧ���ӱ�Ҫ�ij���װ�ã������ͼ�ı�ѡװ����ѡ��������������пո�B ��D ��E ��(������)
(4)�����Ͱ����ڳ��������ͻᷴӦ�����Ȼ�狀͵�����װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ�����ʵ�鷽�������ù�������Ȼ�泥� ��
(5)��װ��C�ij����ܿڴ��ݳ���β�����ܺ�����Ⱦ���������壬��δ����� ��
(1)MnO2��4H����2Cl�� Mn2����Cl2����2H2O
Mn2����Cl2����2H2O
(2)A��B��E
(3)��
(4)ȡһ�����ù����ˮ�ܽ⣬������Һ�ֳ��������Թ��У�������һ���м���NaOH��Һ�����ȣ����ɵ�������ʹʪ��ĺ�ɫʯ����ֽ������˵����NH4+������һ����Һ�м���HNO3�ữ��Ȼ�����AgNO3��Һ�����ְ�ɫ������˵����Cl����ͨ����������֤���ù������Ȼ��
(5)��β��ͨ��ʢ��NaOH��Һ���ձ���
 Mn2����Cl2����2H2O
Mn2����Cl2����2H2O(2)A��B��E
(3)��
(4)ȡһ�����ù����ˮ�ܽ⣬������Һ�ֳ��������Թ��У�������һ���м���NaOH��Һ�����ȣ����ɵ�������ʹʪ��ĺ�ɫʯ����ֽ������˵����NH4+������һ����Һ�м���HNO3�ữ��Ȼ�����AgNO3��Һ�����ְ�ɫ������˵����Cl����ͨ����������֤���ù������Ȼ��
(5)��β��ͨ��ʢ��NaOH��Һ���ձ���
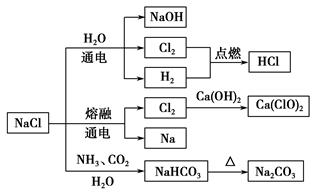
(2)װ��AΪ�����ư�����װ�ã���ѡ�ü�ʯ�ҡ���ʯ�ҡ��ռŨ��ˮ�д������¿�����̣�NH3��H2O NH3��H2O
NH3��H2O NH4+��OH���������ʯ�ҡ���ʯ�ҡ��ռ���ɴ�ʹƽ�����淴Ӧ�����ƶ�������NH3���塣(3)��Aװ���г�����NH3�к���ˮ���������ü�ʯ�Ҹ������ѡ��װ�ã���Fװ���г�����Cl2�к���HCl��H2O��HCl������ñ���ʳ��ˮ���գ�H2O����Ũ�������ա�(5)β������δ��Ӧ���NH3��Cl2����ͨ��ʢ��NaOH��Һ���ձ��н������ա�
NH4+��OH���������ʯ�ҡ���ʯ�ҡ��ռ���ɴ�ʹƽ�����淴Ӧ�����ƶ�������NH3���塣(3)��Aװ���г�����NH3�к���ˮ���������ü�ʯ�Ҹ������ѡ��װ�ã���Fװ���г�����Cl2�к���HCl��H2O��HCl������ñ���ʳ��ˮ���գ�H2O����Ũ�������ա�(5)β������δ��Ӧ���NH3��Cl2����ͨ��ʢ��NaOH��Һ���ձ��н������ա�
 NH3��H2O
NH3��H2O NH4+��OH���������ʯ�ҡ���ʯ�ҡ��ռ���ɴ�ʹƽ�����淴Ӧ�����ƶ�������NH3���塣(3)��Aװ���г�����NH3�к���ˮ���������ü�ʯ�Ҹ������ѡ��װ�ã���Fװ���г�����Cl2�к���HCl��H2O��HCl������ñ���ʳ��ˮ���գ�H2O����Ũ�������ա�(5)β������δ��Ӧ���NH3��Cl2����ͨ��ʢ��NaOH��Һ���ձ��н������ա�
NH4+��OH���������ʯ�ҡ���ʯ�ҡ��ռ���ɴ�ʹƽ�����淴Ӧ�����ƶ�������NH3���塣(3)��Aװ���г�����NH3�к���ˮ���������ü�ʯ�Ҹ������ѡ��װ�ã���Fװ���г�����Cl2�к���HCl��H2O��HCl������ñ���ʳ��ˮ���գ�H2O����Ũ�������ա�(5)β������δ��Ӧ���NH3��Cl2����ͨ��ʢ��NaOH��Һ���ձ��н������ա�
��ϰ��ϵ�д�
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
�����Ŀ

 H��+Cl��+ HClO����ƽ�ⳣ������ʽΪK�� ��
H��+Cl��+ HClO����ƽ�ⳣ������ʽΪK�� ��