��Ŀ����
14����������ƾ��壨Na2S2O3•5H2O��ʽ��248���׳ƺ�������մ���������ˮ�����ܽ�����¶����߶����������������Ҵ�������ʱ�ֽ⣬������������ҵ�Ķ�Ӱ����ʵ����ģ�ҵ�Ʊ���������ƾ���ͨ�������·�������ش��й����⣮�������Ʒ���Na2SO3+S+5H2O=Na2S2O3•5H2O������ʵ���������£�

��1��������Ҵ���ʪ��Ŀ�������������������������Һ��ֽӴ����ӿ췴Ӧ���ʣ�
��2�������в��ܽ���Һ�������ɵ�ԭ�������ɻ�ʹ��������ƾ�����ˮ���ֽ⣮
��3�����ôֲ�Ʒһ��ͨ���ؽᾧ�����ᴿ��
���2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2����Ҫʵ��װ�����£�
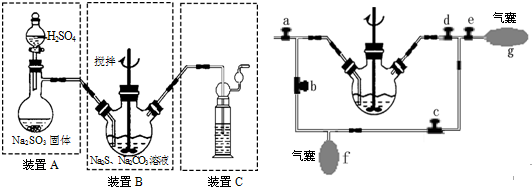
��4��װ��C�����������շ�Ӧ���ɵ�CO2�Ͷ����SO2����ֹ��Ⱦ������
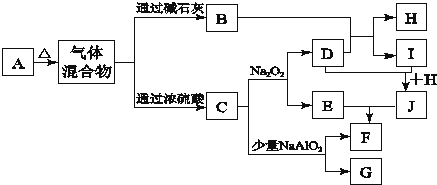
��5��Ϊ�������SO2����װ��B���иĽ���������ͼ��ʾ������A�з�Ӧ�����ر�����b��e����a��c��d����B����Һͨ��SO2��δ��Ӧ��SO2���ռ�������f�У���f�ռ����϶�����ʱ���ٶ���ʱװ��A�з�Ӧ��ֹͣ�����ر�����ac��������bde�����ἷѹf��ʹSO2������ѹ��B����Һ�ٴη�Ӧ��δ��Ӧ��SO2�ֱ��ռ�������g�У��ٽ�g�е����強ѹ��f�У���˷�����ֱ����ȫ��Ӧ��
��6��Ϊ�ⶨ��������ƾ���ֲ�Ʒ�Ĵ��ȣ�ij��ȤС���ȡ5.0�˴ֲ�Ʒ���250mL��Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���25mL 0.01mol/L KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+=3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+S4O62-������ɫ��ȥ�Ұ���Ӳ���ɫʱ����ζ��յ㣮ʵ���������±���
| ����� | 1 | 2 | 3 |
| ����Na2S2O3��Һ��mL�� | 19.98 | 21.18 | 20.02 |
�ڿ������ʵ����ƫ�͵���BD�����ţ���
A����ƿ������ˮ��ϴ
B���ζ���δ��Na2S2O3��Һ��ϴ
C���ζ��յ�ʱ���Ӷ���
D�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ��
���� ��1���Ҵ�������ˮ��������Ҵ���ʪ�����������������������Һ��ֽӴ���
��2����֪��������ƾ�������ֽ⣻
��3����������Ƶ��ܽ�����¶����߶������������¶ȣ������ؽᾧ����ȡ�ֲ�Ʒ��
��4��װ��C��Ҫ��β�����������շ�Ӧ���ɵ�CO2�Ͷ����SO2����ֹ��Ⱦ������
��5���ر�����a��������c�����ἷѹf��ʹSO2������ѹ��B����Һ�ٴη�Ӧ��δ��Ӧ��SO2�ֱ��ռ�������g�У�
��6������ƿ�м���25mL 0.0lmol•L-1KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+�T3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-�T2I-+S4O62-��
��ɵù�ϵʽ��IO3-��6S2O32-���ݴ˼��㣻
A����ƿ������ˮ��ϴ����ʵ����ûӰ�죻
B���ζ���ĩ��Na2S2O3��Һ��ϴ����Na2S2O3��Һ�ᱻϡ�ͣ�
C���ζ��յ�ʱ���Ӷ�����ʹNa2S2O3��Һ���ƫС��
D���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ��ʹ������Na2S2O3��������
��� �⣺��1���Ҵ�������ˮ��������Ҵ���ʪ�����������������������Һ��ֽӴ����ӿ췴Ӧ���ʣ�
�ʴ�Ϊ�����������������������Һ��ֽӴ����ӿ췴Ӧ���ʣ�
��2����֪��������ƾ�������ֽ⣬�����ɻ�ʹ�����������ˮ���ֽ⣬���Դ������������Һ�з�������ʱ���ܽ���Һ�������ɣ�
�ʴ�Ϊ�����ɻ�ʹ�����������ˮ���ֽ⣻
��3����������Ƶ��ܽ�����¶����߶������������¶ȣ������ؽᾧ����ȡ�ֲ�Ʒ��
�ʴ�Ϊ���ؽᾧ��
��4��װ��C��Ҫ��β�����������շ�Ӧ���ɵ�CO2�Ͷ����SO2����ֹ��Ⱦ������
�ʴ�Ϊ�����շ�Ӧ���ɵ�CO2�Ͷ����SO2����ֹ��Ⱦ������
��5���ر�����ac��������bde�����ἷѹf��ʹSO2������ѹ��B����Һ�ٴη�Ӧ��δ��Ӧ��SO2�ֱ��ռ�������g�У�
�ʴ�Ϊ��ac��bde��
��6������ƿ�м���25mL 0.0lmol•L-1KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+�T3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-�T2I-+S4O62-��
��ɵù�ϵʽ��IO3-��6S2O32-��
1mol 6mol
0.025L��0.0lmol•L-1 n��S2O32-��
��n��S2O32-��=0.0015mol��
������ʵ����������ϴ���ȥ��
����250mL�����������Һ����������Ƶ����ʵ���Ϊ0.0015mol��$\frac{250}{\frac{1}{2}��19.98+20.02��}$=0.01875mol��
����������Ƶ�����Ϊ0.01875mol��248g/mol=4.65g��
��ò�Ʒ�Ĵ�����$\frac{4.65g}{5.0g}$��100%=93%��
A����ƿ������ˮ��ϴ����ʵ����ûӰ�죬���Ȳ��䣬��A��ѡ��
B���ζ���ĩ��Na2S2O3��Һ��ϴ����Na2S2O3��Һ�ᱻϡ�ͣ����Բ����������Ƶ�����ƫС���ʴ���ƫС����Bѡ��
C���ζ��յ�ʱ���Ӷ�����ʹNa2S2O3��Һ���ƫС�����������������Ƶ�����ƫ�ʴ���ƫ��C��ѡ��
D���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ��ʹ������Na2S2O3�����������ԭ��Һ�е���������Ƶ�����ƫС����ƫС����Dѡ��
�ʴ�Ϊ��93%��BD��
���� ����ͨ����ȡNa2S2O3•5H2O��ʵ������������������Ʊ���������ơ�����ʵ����������ӷ���ʽ����д�����ʴ��ȵļ��㡢�ζ��������ȣ���Ŀ�Ѷ��еȣ���ȷʵ���������Ƽ�������ʵ������ǽ����Ĺؼ��������ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | Z��N�ļ����ӵĻ�ԭ�ԣ�Z2-��N- | |
| B�� | Y2X��Y2X2���Ǽ��������� | |
| C�� | Z��X�γɵĻ������Ӧ��ˮ����һ����ǿ�� | |
| D�� | ZԪ�صķǽ����Ա�NԪ�صķǽ�����ǿ |
| ����ʽ | ��� | �ȷֽ��¶� | �۵� | �ܽ��� |
| CO��NH2��2•H2O2 | ��ɫ���� | 45�� | 75-85�� | ������ˮ���л��ܼ� |
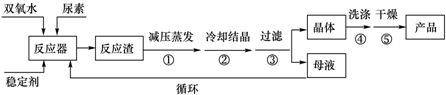
��ش��������⣺
��1����Ӧ���ļ��ȷ�ʽ��ˮԡ���ȣ���Ӧ�¶���������ʵ��¶��½��У��¶Ȳ��ܹ��ߵ�ԭ�����¶ȹ��ߣ���Ʒ�ֽ⣬��ʹ�������������ͣ��¶�Ҳ���ܹ��͵�ԭ�����¶ȹ��ͣ���Ӧ����̫�����ҷ�Ӧ��ϵ������Ҫ���Ĵ���������
��2��������ĸҺ�з����H2O2�����أ��ɲ��õIJ����Ǽ�ѹ���ᾧ��
��3���ɷ�������ȡ���������صķ����ǣ����ø�Ũ��˫��ˮˮ��Һ��������ˮ���ع����Ͻ��з�Ӧ��ˮ�ͷ�Ӧ��ͨ����̬����ȥ���õ�����Ĺ��������ز�Ʒ��
�Ƚϸɷ���ʪ�����ֹ��գ�����Ϊ�ɷ����յ��ŵ��ǣ����̶̣����ռ����һ�㼴�ɣ����ɷ����յ�ȱ���ǣ�˫��ˮŨ�ȸ߾���Ч��ͣ��豸���ӵȣ�������㼴�ɣ���ʪ�����յ��ŵ��ǣ���Ũ��˫��ˮ������Ч��ߣ��豸�����ڴﵽ��ĸҺ��ѭ��ʹ�õȣ�������㼴�ɣ���
��4��ȷ��ȡ0.6000g��Ʒ��250mL��ƿ�У�����������ˮ�ܽ⣬�ټ�1mL 6mol•L-1H2SO4����0.1000mol•L-1KMnO4����Һ�ζ����յ�ʱ����20.00mL��������KMnO4��Һ����Ӧ�������Ʒ��CO��NH2��2•H2O2����������Ϊ78.3%�����������С�����һλ����

��֪����Ksp��CaF2��=1.46��10-10��Ksp��CaC2O4��=2.34��10-9��
| �������� | ��ʼ������pH | ������ȫ��pH |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Al3+ | 3.0 | 5.0 |
| Ni2+ | 6.7 | 9.5 |
��1�������顱��Ŀ��������Ӵ�������ӿ췴Ӧ���ʣ�������Ľ����ʣ�
��2����������������ͬ���ڲ�ͬ�¶��¶Է����������С������������������ʱ��仯��ͼ����������������¶���ʱ��ֱ�Ϊc������ĸ����
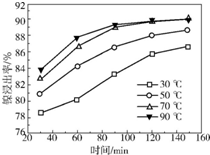
a.30�桢30min b.90�桢150min c.70�桢120min d.90�桢120min
��3��֤����������������Ni2+�Ѿ�������ȫ��ʵ�鲽�輰�����Ǿ��ã����ϲ���Һ�м����μӣ�NH4��2C2O4��Һ�������ٲ��������������������Ѿ���ɣ���������������õ��Ļ������ˣ����ù�����75%�Ҵ���Һϴ�ӡ�110���º�ɣ��ò��������壮��75%�Ҵ���Һϴ�ӵ�Ŀ����ϴȥ��NH4��2SO4���ʡ����ں�ɡ����ٲ�����������ʧ��
��4���ڳ������������У�Ӧ�ȼ���H2O2�������ټ�������������pHֵ�ķ�ΧΪ5.0��pH��6.7����2���м�������NH4F��Һ�������dz�ȥ����Ca2+��
��5�����õ��IJ���������������м�����320��ֽ�������Ƶõ�����������д�����Ʊ����̵Ļ�ѧ����ʽ��NiC2O4•2H2O$\frac{\underline{\;320��\;}}{\;}$Ni+2CO2��+2H2O��
��6����֪����������������������Ϊ5.9%����100kg�������������Ƶ�18.3kg���������壨Ni��59��C��12��H��1��O��16����
| A�� | $\frac{1000V��}{22400+36.5V}$ mol/L | B�� | $\frac{V��}{22400}$ mol/L | ||
| C�� | $\frac{V��}{22400+36.5}$ mol/L | D�� | $\frac{V}{22.4}$ mol/L |
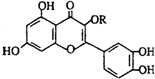 ��Ȼά����P���ṹ��ͼ���������ڻ��������У�����һ��Ӫ��������������ά����P������������ǣ�������
��Ȼά����P���ṹ��ͼ���������ڻ��������У�����һ��Ӫ��������������ά����P������������ǣ�������| A�� | 1mol���л���һ�������¿��Ժ�5mol��ˮ��Ӧ | |
| B�� | ���л���ķ���ʽΪC15H8O7R | |
| C�� | 1mol���л���һ���������������8 mol H2 | |
| D�� | 1molά����P���Ժ�4molNaOH��Ӧ |
| A�� | NO2 | B�� | NO | C�� | N2O | D�� | N2O3 |
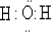 ��
��
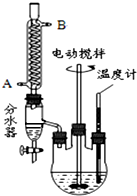 ������ܻ����ڱ�����������������ڱ�������������������Ũ���Ṳ���·�Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽ��װ��ͼ������װ��ʡ�ԣ���ͼ��
������ܻ����ڱ�����������������ڱ�������������������Ũ���Ṳ���·�Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽ��װ��ͼ������װ��ʡ�ԣ���ͼ�� +C4H9OH$\stackrel{H_{2}SO_{4}}{��}$
+C4H9OH$\stackrel{H_{2}SO_{4}}{��}$ 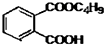 $��_{C_{4}H_{9}OH}^{H_{2}SO_{4}}$
$��_{C_{4}H_{9}OH}^{H_{2}SO_{4}}$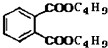
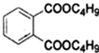 +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$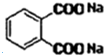 +2CH3CH2CH2CH2OH��
+2CH3CH2CH2CH2OH��