��Ŀ����
��̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������д���Ӧ�á�
��֪����̼������������Һ��Ӧ�Ļ�ѧ����ʽ���£�
Na2CO4 +H2SO4 ��Na2SO4 +H2O2 + CO2�� 2H2O2 ��2H2O+ O2��
Ϊ�ⶨ�ѱ��ʵĹ�̼����(��̼����)�Ĵ��ȣ������ͼ��ʾ��ʵ�飺QΪ���������õĵ��Ե������뷴Ӧ��������ﷴӦ������ȡһ��������Ʒ�������������̷������У���ͼ��װ��ʵ��װ�ã���Һ©���Ļ�������ϡH2SO4���������С�
��1��Q�ڷ�����Ӧ���ɵ�����Ϊ__________��
����Ʒ�м��������������̵�Ŀ����________________________________��
����a������_____________________��
��2��Ϊ�����Ӧʱ������������������ϡH2SO4ǰ����
�ر�_______(��K1��K2��K3����ͬ)����______��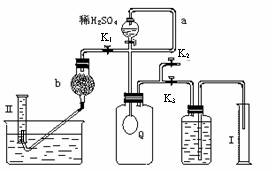
��3����������Ӧֹͣ����K1��K2��K3���ڹر�״̬��Ȼ���ȴ�K2���ٻ�����K1����ʱ�ɹ۲쵽��������___________________��b��װ�ļ�ʯ�ҵ�������__________________��Ϊ��Ҫ������K1��������_____________________________��
��4��ʵ�����ʱ����ͲI����xmLˮ����Ͳ�����ռ���ymL����(��������������㵽��״��)�����̼���ƵĴ�����____________
��5��ijͬѧʵ���õĹ�̼���ƵĴ��ȳ���100%������Ϊ���ܵ�ԭ����_______
A������������Q�͵������У�δȫ��������Ͳ��
B����Ͳ�����ʱ����ͲҺ�����ˮ��Һ��
C���Ҳ���Ͳ��ʹ�Һ����ƿ���ӵ����ڵ�Һ��û�м������x
D�����������ֵx��yû�п۳��μӵ���������
��1��CO2��O2����1�֣���ʹ˫��ˮ��ȫ�ֽ��������1�֣���ƽ���Һ©���ϡ��µ�ѹǿ��ʹϡH2SO4˳�����¡���2�֣�
��2��K1��K2��K3����2�֣� ��3������Q������С����ർ�����������ɡ���2�֣�
���ն�����̼��1�֣��������������٣�ʹCO2��������ա���2�֣�
��4��[12200y/(53x-37y)]%����3�֣�����5��BC ��3�֣�

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�