��Ŀ����
�Ը������������Ĺ�ҵ��ҺΪԭ�������������Ĺ������£����ֲ����������ԣ�����.�ӷ�Һ���ᴿ���ᾧ��FeSO4��7H2O��
��.��FeSO4��7H2O���Ƴ���Һ��
��.FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3����Һ��
��.����Һ���ˣ���90����ˮϴ�ӳ����������õ�FeCO3���塣
��.����FeCO3���õ�Fe2O3���塣
��֪��NH4HCO3����ˮ�зֽ⡣
��1�����У�����������м��ȥ��Һ�е�Fe3+���÷�Ӧ�����ӷ���ʽ��_________________��
��2�����У����һ�������ᡣ���û�ѧƽ��ԭ���������������_____________��
��3�����У�����FeCO3�����ӷ���ʽ��_____________����FeCO3��Һ��ʱ�䱩¶�ڿ����У����в��ֹ�������Ϊ���ɫ���ñ仯�Ļ�ѧ����ʽ��_____________��
��4�����У�ͨ������![]() ���жϳ����Ƿ�ϴ�Ӹɾ�������
���жϳ����Ƿ�ϴ�Ӹɾ�������![]() �IJ�����_____________��
�IJ�����_____________��
��5����֪����FeCO3�Ļ�ѧ����ʽ��4FeCO3+O2![]() 2Fe2O3+4CO2��������464.0 kg��FeCO3,�õ�316.8 kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������_________ kg����Ħ������/g��mol-1:FeCO3 116 Fe2O3 160 FeO 72��
2Fe2O3+4CO2��������464.0 kg��FeCO3,�õ�316.8 kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������_________ kg����Ħ������/g��mol-1:FeCO3 116 Fe2O3 160 FeO 72��
��1��Fe+2Fe3+====2Fe2+
(2)�������ᣬH+Ũ������ʹFe2++2H2O![]() Fe(OH)2+2H+��ƽ�����淴Ӧ�����ƶ����Ӷ�����FeSO4��ˮ��
Fe(OH)2+2H+��ƽ�����淴Ӧ�����ƶ����Ӷ�����FeSO4��ˮ��
��3��Fe2++![]() ====FeCO3��+H+
====FeCO3��+H+
4FeCO3+6H2O+O2====4Fe��OH��3��+4CO2
(4)ȡ����ϴ�Ӻ����Һ�����Թ��У��μ��ữ��BaCl2��Һ�����ް�ɫ���������������ϴ�Ӹɾ�
��5��288.0
�����������ۺϿ���Fe��������ʡ����ӷ���ʽ����д����ѧ����ʽ����д��ˮ����ɵ����á����Ӽ��鼰��ѧ�������������
��2��FeSO4+2H2O![]() Fe(OH)2+H2SO4,����H2SO4��ƽ�����ƣ�����ˮ�⡣
Fe(OH)2+H2SO4,����H2SO4��ƽ�����ƣ�����ˮ�⡣
��3�������ɫ����ʾ����Fe(OH)3��
��4��![]() ���飺˵�����裬ָ���Լ��������ۡ�
���飺˵�����裬ָ���Լ��������ۡ�
��5�����Ʒ��FeO��Fe2O3���ʵ����ֱ�Ϊa mol��b mol��
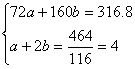 ���:b=1.8 mol
���:b=1.8 mol
m(Fe2O3)=1.8 mol��160 g��mol-1=288.0 g��

 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д� ����
����
 I �ӷ�Һ���ᴿ���ᾧ��FeSO4��7H2O��
I �ӷ�Һ���ᴿ���ᾧ��FeSO4��7H2O�� ��Һ���Թ�����
��Һ���Թ����� ��Һ��ϣ��õ���
��Һ��ϣ��õ��� ����Һ
����Һ IV ����Һ���ˣ���90��C��ˮϴ�ӳ����������õ�
IV ����Һ���ˣ���90��C��ˮϴ�ӳ����������õ�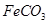 ����
���� ����
����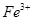 ���÷�Ӧ�����ӷ���ʽ��
���÷�Ӧ�����ӷ���ʽ��
 ���жϳ����Ƿ�ϴ�Ӹɾ�������
���жϳ����Ƿ�ϴ�Ӹɾ������� ��֪����
��֪���� ��������464.0kg��
��������464.0kg�� ����ò�Ʒ��
����ò�Ʒ�� ��������
kg��Ħ������/g��
��������
kg��Ħ������/g�� ��
�� ��
��