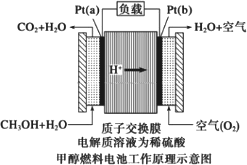
��Ŀ����
��11�֣�ȼ���ڵ����ֱ���������������ܵ�װ�ý�ȼ�ϵ�أ�����һ�ָ�Ч����Ⱦ�����͵�ء�ȼ�ϵ������ȼ�Ͽ�����������Ҳ����������ȼ�ϣ���״����µȡ��·��ӣ�NH2��NH2��������������ȼ�����ɵ�����ˮ���ش��������⣺
��1�������¡�������KOH��Һ��ɼ���ȼ�ϵ�أ���д���õ�ظ����ĵ缫��Ӧʽ��
����ָ���������Һ��OH-������ ���ƶ���
��2���õ��뷽��ʽ��ʾ�µ�ˮ��Һ�ʼ��Ե�ԭ�� ��
��3������ǿ��ԭ��������������Ӧʱ�ų��������ȣ��磺
N2H4(l)��2H2O2(l)��N2(g)+4H2O(g) ��H����642.2 kJ/mol
��ˣ��¿�����Ϊ����ƽ���������������Ϣ������Ϊ�Ƿ����ͨ���ı䷴Ӧ�������ɵ�����ˮ��������ȡ�� ��˵��ԭ�� ��
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�